
SUPER HAIR GROWTH COMBO SAMPLER – $1400 VALUE FOR $575 (SAVE 59%)
March 12, 2024
SIRT-1: Longevity Gene Activator – New! Shipping Now!
May 14, 2024NRF2 ACTIVATOR (Antioxidant Defense) – New!
$275.00
The NRF2 Activator represents a significant advancement in antioxidant defense mechanisms. This innovative formulation is designed to enhance the body’s natural ability to combat oxidative stress, thereby promoting overall health and wellness. By activating the NRF2 pathway, this product facilitates the expression of critical antioxidant enzymes, which play a vital role in neutralizing free radicals. Clinical studies indicate that the NRF2 Activator can contribute to improved cellular resilience and longevity. As such, it serves as a valuable addition to any health regimen aimed at fostering enhanced protective responses against environmental and physiological stressors. Interstellar Blend™: Ultimate Nrf2 Activator for Antioxidant Defense
Discover Interstellar Blend™, a groundbreaking 200:1 concentration Nrf2 activator designed as the master regulator of antioxidant defense. This premium herbal supplement combats oxidative stress, supports redox homeostasis, detoxification, inflammation reduction, autophagy, mitochondrial function, DNA repair, and metabolism reprogramming. Ideal for addressing Nrf2 signaling in obesity, antioxidant response, anti-inflammation gene expression, glutathione regulation, cytoprotective genes, cancer herbal remedies, and cellular defense.
Formulated with a potent synergy of natural ingredients, Interstellar Blend™ harnesses the power of 5,7-dihydroxychromone, Acetyl-Cysteine, Allicin, alpha-lipoic acid, Andrographolide, Astaxanthin, Astilbin from Engelhardtia roxburghiana, Baicalein, Baicalin, Blueberry anthocyanins, Brassica juncea, Brassica oleracea, Brassica rapa, Butein, Caffeic acid, Calendula officinalis, Capsaicin, Carnosic acid, Carnosol, Carotene, Catechin, Chlorogenic Acid, Cichoric acid, CORIANDRUM SATIVUM, Crocin, Curcumin, Cyanidin-3-O-glucoside, Dandelion Extract, Danshen Extract, Dihydromyricetin, Diosgenin, Echinacea purpurea, Echinatin, Eggplant extract, Elderberries extract anthocyanin, Ellagic acid, Epigallocatechin-3-gallate (EGCG), Ethyl ferulate, Eupatolide, Ferulic Acid, Gallic acid, Ganodermanondiol, Garcinone D, Genistein, Ginkgo leaves Flavone Glycoside, Glucoraphanin, Glutathione, Glycyrrhizinic Acid, Goniothalamin, Grape seed procyanidins, Green Tea Polyphenols, Guggulsterone, Hinokitiol, Hydroxytyrosol, Hyperoside, Isoorientin, Kaempferol, Kinsenoside, L-Epicatechin, Lutein, Luteolin, lycopene, Myricetin, Naringenin, Naringin, Neoxanthin, Notoginsenoside R2, Oleanolic acid, Olive Leaf Extract Oleurope, Origanum vulgare extract, P-coumaric acid, Perilla frutescens extract, Petroselinum crispum extract, phenethyl isothiocyanate, Phloretin, Piper methysticum, Polygonum cuspidatum (Japanese knotweed), Pomegranate extract, Potato extract, Procyanidin B2, protocatechuic acid, Purple sweet potato extract, Quercetin, Raphanus sativus, Red wine extract, Rehmapicrogenin, Resveratrol, Retinoic acid, Rosmarinic acid, Salidroside, Silibinin, Silymarin, Soy isoflavone, Sulforaphane, Tangeretin, Taxifolin, tert-Butylhydroquinone, Turnips extract, Ursolic Acid, Vitamin D, Withania Somnifera, Wogonin, Wogonoside, Xanthohumol, Zeaxanthin, Zerumbone, and β-Carotene.
Experience enhanced cellular defense and vitality with Interstellar Blends—your natural shield against oxidative stress and inflammation. Boost Nrf2 pathway activation today for optimal health!
INTRODUCING
INTERSTELLAR BLEND™
Nrf2
activator
Master Regulator of Antioxidant Defense
200:1 Concentration





In the vast expanse of the cosmic plane, where stardust weaves tales arcane,
Dwells NRF2, a sentinel unseen, a knight in proteins’ radiant sheen.
Guardian of health, bearer of time, in the body’s symphony, its rhythm chimes.
The interstellar blend, a dance so grand, born from life’s ancient hand,
NRF2, the master regulator, against the oxidative desecrator.
Fights the invaders that inflame, defends our cells in life’s fierce game.
When toxins knock and stress does call, it listens to that daunting squall,
Leaping to our DNA, a wordless poem it begins to play.
Commands the genes, with orders tight, to rise, to fight the endless night.
A host of enzymes, warriors bold, against free radicals, they hold.
Combatting the invaders that dare, to damage cells in need of care.
A shield, a sword, a watchful gaze, in the dance of life, it leads the maze.
For in this dance so intricate, it slows the hands of time’s swift gait,
Promoting longevity’s sweet song, where health and harmony belong.
Thus, in the interstellar blend, with NRF2, our life extends.
An epic tale of life’s defense, in the realm of science immense,
A master regulator’s role, in the cosmic, life’s noble scroll.
NRF2, our silent guard, in life’s ballet, its role, regard.
Nrf2 is a transcription factor that serves as a master regulator of cytoprotective mechanisms. It activates the transcription of over 500 genes involved in detoxification, antioxidant defense, anti-inflammatory responses, mitochondrial function, and autophagy. By increasing the expression of these genes, Nrf2 enhances the cell’s ability to protect itself from oxidative stress, inflammation, and toxic insults. This activation of cytoprotective mechanisms is crucial for maintaining cellular homeostasis and preventing the development of chronic inflammatory diseases. Various health-promoting factors, such as phenolic antioxidants, omega-3 fatty acids, and certain phytochemicals, can raise Nrf2 activity, highlighting the importance of a balanced and nutrient-rich diet in promoting cellular health and resilience.
Nrf2 prevents obesity through various mechanisms.
Firstly, Nrf2 activation leads to the upregulation of antioxidant enzymes and proteins, which helps to counteract oxidative stress and reduce adipocyte differentiation and adipose tissue expansion. This prevents excessive accumulation of adipose tissue and the development of obesity.
Secondly, Nrf2 activation has anti-inflammatory effects by inhibiting the production of proinflammatory cytokines and chemokines. This reduces chronic inflammation in adipose tissue, which is closely associated with obesity.
Thirdly, Nrf2 activation improves insulin signaling and sensitivity, thereby reducing insulin resistance, which is a major pathological component of obesity.
Fourthly, Nrf2 activation increases energy expenditure and decreases food intake, leading to a reduction in body weight and fat gain.
Finally, Nrf2 activation can regulate the expression of genes involved in lipid metabolism, glucose utilization, and protein synthesis, which helps to maintain metabolic homeostasis and prevent obesity. Overall, Nrf2 activation plays a critical role in combating obesity by regulating oxidative stress, inflammation, insulin resistance, energy balance, and metabolic processes.
The role of Nrf2 in cellular survival is multifaceted and involves several mechanisms. Here are the key roles of Nrf2 in promoting cellular survival:
1. Activation of antioxidant defense: Nrf2 plays a crucial role in activating the cellular antioxidant defense system. It up-regulates the expression of genes encoding antioxidant enzymes, such as glutathione peroxidase, glutathione reductase, and peroxiredoxin. These enzymes help neutralize reactive oxygen species (ROS) and protect cells from oxidative damage, thereby promoting cellular survival.
2. Detoxification of harmful substances: Nrf2 activates genes encoding phase II detoxification enzymes, including glutathione S-transferases and NAD(P)H: quinone oxidoreductase 1. These enzymes are involved in the detoxification and elimination of harmful substances, such as carcinogens and reactive metabolites. By enhancing the detoxification capacity of cells, Nrf2 helps protect against cellular damage and promotes survival.
3. Maintenance of redox balance: Nrf2 regulates the expression of genes involved in the synthesis, regeneration, and utilization of glutathione, a key molecule involved in maintaining cellular redox balance. Glutathione acts as a potent antioxidant and helps maintain the cellular redox state. By regulating the expression of genes involved in glutathione metabolism, Nrf2 ensures the availability of this important molecule for cellular survival.
4. Modulation of apoptosis signaling pathways: Nrf2 has been shown to modulate apoptosis signaling pathways, which play a critical role in cellular survival. It has been observed that Nrf2 can protect against apoptosis induced by various stimuli. Additionally, Nrf2 has been found to regulate the sensitivity of death receptor signals, further contributing to cellular survival.
5. Protection against oxidative stress-induced damage: Nrf2 confers protection against oxidative stress-induced cellular damage. It activates genes involved in cellular defense against oxidative stress, including those encoding antioxidant enzymes and detoxification enzymes. By enhancing the cellular antioxidant capacity and detoxification mechanisms, Nrf2 helps protect cells from oxidative damage and promotes their survival.
6. Regulation of cell signaling pathways: Nrf2 has been found to regulate various cell signaling pathways involved in cellular survival. It interacts with other transcription factors and signaling molecules to modulate gene expression and cellular responses. For example, Nrf2 has been shown to interact with the PERK pathway, which is involved in cell survival during endoplasmic reticulum stress.
In summary, Nrf2 plays a crucial role in cellular survival by activating antioxidant defense mechanisms, promoting detoxification of harmful substances, maintaining redox balance, modulating apoptosis signaling pathways, protecting against oxidative stress-induced damage, and regulating cell signaling pathways. These functions collectively contribute to the overall survival and well-being of cells.
Nrf2 plays a crucial role as a master regulator of mammalian aging by regulating multiple pathways involved in aging and age-related diseases. Activation of Nrf2 can extend lifespan, improve healthspan, and protect against age-related diseases by reducing oxidative stress, inflammation, and cellular senescence.
Nrf2 regulates multiple pathways involved in aging and age-related diseases, including:
– Antioxidant response
– Redox homeostasis
– Detoxification
– Inflammation
– Autophagy
– Mitochondrial function
– DNA repair
INGREDIENTS & SCIENCE
5,7-dihydroxychromone
5,7-dihydroxychromone activates the Nrf2 signaling pathway, enhancing antioxidant defenses and reducing oxidative stress. This compound shows potential neuroprotective effects, supports cognitive function, and may mitigate inflammation and cancer progression, highlighting its significance in health and disease management.
5,7-Dihydroxychromone activates Nrf2/ARE, offering neuroprotection against oxidative stress.
5,7-Dihydroxychromone protects SH-SY5Y cells from 6-OHDA neurotoxicity by activating the Nrf2/ARE pathway, increasing antioxidant enzymes, reducing ROS, and preventing neuronal cell death in Parkinson’s disease.
Acetyl-Cysteine
Acetyl-Cysteine boosts NRF2 activity, enhancing cellular antioxidant defenses and glutathione production. It reduces oxidative stress, inflammation, and supports detoxification. Evidence suggests benefits for respiratory health, liver protection, and mitigating neurodegenerative conditions. Its role in NRF2 activation aids cellular resilience, offering potential therapeutic effects against chronic diseases and oxidative damage.
N-Acetyl-Cysteine enhances Nrf2 antioxidant gene expression in asthenoteratozoospermia men.
NAC oral supplementation boosts Nrf2 expression, reducing oxidative stress and improving semen quality in asthenoteratozoospermia men. Enhances sperm motility and morphology, promoting male fertility health.
I-152 activates Nrf2 and ATF4 signaling, enhancing glutathione production.
I-152, combining NAC and cysteamine, boosts GSH levels via Nrf2/KEAP1 pathway activation. At high doses, it activates ATF4, influencing cell proliferation and survival.
S-allylmercapto-N-acetylcysteine activates Nrf2, reducing pulmonary fibrosis in mice.
ASSNAC-Na inhalation improves pulmonary fibrosis in mice by reducing collagen deposition, oxidative stress, inflammation, and fibroblast differentiation via Nrf2/NOX4, NF-κB, and TGF-β1/Smad2/3 pathways.
Allicin
Allicin activates the NRF2 pathway, enhancing cellular antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, anticancer, and cardiovascular protective effects. Studies indicate allicin’s role in supporting detoxification, protecting against neurodegenerative diseases, and regulating immune responses by promoting NRF2-mediated antioxidant gene expression and inhibiting pro-inflammatory pathways.
Allicin activates Nrf2-HO-1, inhibits NLRP3, protecting cardiomyocytes from LPS injury.
Allicin protects cardiomyocytes from LPS-induced injury by activating Nrf2/HO-1 and inhibiting NLRP3 pathways, reducing inflammation, apoptosis, and oxidative stress while enhancing cell viability.
Allicin reduces oxidative stress, inflammation, activates Nrf2, and protects endothelial cells.
Allicin attenuates LPS-induced vascular injury by reducing oxidative stress and inflammation in HUVECs. It modulates Nrf2 activation, protecting blood vessels from LPS-induced inflammatory damage and vascular injury.
alpha-lipoic acid
Alpha-lipoic acid activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It offers neuroprotective, anti-inflammatory, and anti-aging effects, supports glucose metabolism, and improves insulin sensitivity. Studies show alpha-lipoic acid protects against neurodegenerative diseases, cardiovascular issues, and metabolic disorders by promoting cellular detoxification and antioxidant gene expression via NRF2 activation.
Alpha-lipoic acid (ALA) protects against MTX-induced liver injury by activating Nrf2/HO-1 pathway, reducing inflammation, oxidative stress, apoptosis, and suppressing HSC activation, improving liver function.
Alpha-lipoic acid activates Nrf2, protects endothelial cells from TNF-α dysfunction.
Oxidized alpha-lipoic acid activates Nrf2, protecting HUVECs from TNF-α-induced dysfunction. It inhibits NF-κB activation and apoptosis, demonstrating its antioxidant role via electrophilic action.
Alpha-lipoic acid activates Nrf2, inhibiting IL-8 expression in H. pylori-infected cells.
Alpha-lipoic acid activates the Nrf2/HO-1 pathway, reducing oxidative stress, IL-8 production, and ROS in H. pylori-infected cells, potentially preventing H. pylori-induced gastric diseases.
Andrographolide
Andrographolide activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It offers anti-inflammatory, anticancer, and hepatoprotective effects, and supports cardiovascular health. Studies show andrographolide protects against neurodegenerative diseases, diabetes, and metabolic disorders by promoting detoxification, regulating inflammatory pathways, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Andrographolide activates Nrf2/HO-1, suppresses microglial activation in Aβ42 exposure.
Andrographolide activates the Nrf2/HO-1 pathway, reduces amyloid beta-induced inflammation, and inhibits NF-κB activity in microglial cells, suggesting potential therapeutic benefits for Alzheimer’s disease.
Andrographolide and plant endophyte extracts regulate Nrf2 transcription factor activation.
ORX 41, a Phomopsis sp. dichloromethane extract, shows strong Nrf2-inducing properties and potential anti-inflammatory effects. Compounds like cytochalasin H/J may contribute to its antioxidant activity.
Andrographolide activates p38 MAPK, ERK, inducing Nrf2 and HO-1 in astrocytes.
Andrographolide activates Nrf2 and HO-1 in astrocytes, reducing oxidative stress and neuroinflammation. Its biphasic regulation involves p38 MAPK and ERK signaling, suggesting neuroprotective potential.
Astaxanthin
Astaxanthin activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It offers neuroprotective, anti-inflammatory, and cardioprotective effects, and supports skin health. Research shows astaxanthin protects against neurodegenerative diseases, cardiovascular disorders, and metabolic issues by promoting detoxification, modulating immune responses, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Astaxanthin as a Modulator of Nrf2, NF-κB, and Their Crosstalk
Astaxanthin modulates Nrf2 and NF-κB pathways, reducing oxidative stress and inflammation. It enhances antioxidant defenses, supporting disease prevention where oxidative stress and inflammation are key factors.
Astaxanthin activates Nrf2/HO-1, enhancing autophagy and inhibiting ferroptosis.
Astaxanthin (ASX) protects against acetaminophen-induced liver injury by activating the Nrf2/HO-1 pathway, reducing oxidative stress, autophagy, and ferroptosis. HMSN@ASX nanoparticles enhance targeted liver delivery.
Astilbin from Engelhardtia roxburghiana
Astilbin from Engelhardtia roxburghiana activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, antidiabetic, and hepatoprotective effects. Studies show astilbin supports immune regulation, protects against liver damage, and combats metabolic disorders by promoting detoxification, modulating inflammatory pathways, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Astilbin activates Nrf2, reducing ROS and VEGF expression in psoriasis.
Astilbin induces Nrf2 nuclear translocation, reducing ROS accumulation and VEGF expression, and inhibiting HaCaT cell proliferation, showing potential for antioxidant and anti-inflammatory effects.
Baicalein
Baicalein activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It offers neuroprotective, anti-inflammatory, and anticancer effects, and supports cardiovascular and liver health. Research shows baicalein protects against neurodegenerative diseases, cardiovascular issues, and metabolic disorders by promoting detoxification, regulating inflammation, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Baicalein and baicalin activate Nrf2 via ERK1/2, PKC, reducing liver injury.
Baicalein protects against acetaminophen-induced hepatotoxicity by activating the Nrf2 pathway, reducing oxidative stress, and inducing antioxidant gene expression. It involves ERK1/2 and PKC-mediated Nrf2 phosphorylation, preventing liver injury.
Baicalein activates PERK/Nrf2, reducing liver oxidative stress and apoptosis.
Baicalein alleviates oxidative stress and apoptosis in diabetic mice by modulating the PERK/Nrf2 pathway, enhancing Nrf2 activation, and regulating HO-1 and CHOP expression, suggesting potential benefits for diabetes management.
Baicalein activates Nrf2/HO-1, involving PKCα, PI3K/AKT, protecting against neurotoxicity.
Baicalein protects against Parkinson’s disease by activating the Nrf2/HO-1 pathway, reducing oxidative stress, and involving PKCα and PI3K/AKT signaling. It enhances antioxidant defense and prevents 6-OHDA-induced neurotoxicity.
Baicalin
Baicalin activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It offers anti-inflammatory, neuroprotective, and hepatoprotective effects, and supports cardiovascular health. Research shows baicalin protects against neurodegenerative diseases, liver injury, and metabolic disorders by promoting detoxification, modulating inflammation, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Baicalin activates Nrf2-HO1, inhibits NF-κB, reducing LPS-induced oxidative stress.
Baicalin protects IPEC-J2 cells from LPS-induced oxidative stress by activating the Nrf2-HO1 pathway and inhibiting NF-κB signaling, enhancing antioxidant enzyme activity and reducing inflammation.
Blueberry anthocyanins
Blueberry anthocyanins activate the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. They offer neuroprotective, anti-inflammatory, and cardiovascular benefits. Research shows blueberry anthocyanins protect against neurodegenerative diseases, metabolic disorders, and heart disease by promoting detoxification, modulating inflammation, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Blueberry anthocyanins activate Nrf2, offering protection against cardiovascular disorders, neurodegenerative diseases, cancer, inflammation, and age-related macular degeneration. They enhance antioxidant defenses in diabetic retinal tissue.
Brassica juncea
Brassica juncea activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It offers anti-inflammatory, anticancer, and hepatoprotective effects. Studies show Brassica juncea protects against liver damage, metabolic disorders, and cardiovascular diseases by promoting detoxification, modulating inflammation, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Glucosinolates from Brassica juncea activate Nrf2/ARE, providing protection against metabolic syndromes, inflammation, oxidative stress, and cancer. They enhance detoxification and antioxidant defenses.
Brassica oleracea
Brassica oleracea activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It offers anti-inflammatory, anticancer, and cardiovascular benefits. Studies show Brassica oleracea supports metabolic health, protects against neurodegenerative diseases, and promotes detoxification by inducing NRF2-mediated antioxidant gene expression and regulating inflammation for cellular protection.
Sulforaphane and nutrigenomic Nrf2 activators offer promising, but varied, clinical outcomes.
Sulforaphane, a potent Nrf2 activator, surpasses curcumin, silymarin, and resveratrol in bioavailability and cytoprotective gene expression, enhancing cellular defense and chemoprevention.
Sulforaphane, found in Brassica oleracea, activates Nrf2, protecting against cancer, inflammation, cardiovascular diseases, neurodegenerative disorders, diabetes, and respiratory diseases.
Ethanolic extract of Brassica oleracea var. acephala activates Nrf2, induces apoptosis, inhibits NF-κB inflammation, and reduces PC3 prostate cancer cell viability.
Brassica rapa
Brassica rapa activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It offers anti-inflammatory, anticancer, and cardioprotective effects. Research shows Brassica rapa supports metabolic health, protects against neurodegenerative diseases, and promotes detoxification by inducing NRF2-mediated antioxidant gene expression and modulating inflammation for cellular protection.
Brassicaphenanthrene A from Brassica rapa activates Nrf2, enhancing HO-1 expression, GSH levels, and offers neuroprotection via PI3K/Akt and JNK pathways.
Arvelexin inhibits IκB kinase, suppressing NF-κB pro-inflammatory gene expression in Brassica rapa.
Brassica rapa, through arvelexin, activates Nrf2, reducing inflammation, septic shock, and oxidative stress, enhancing cytoprotective defenses.
Butein
Butein activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It offers anti-inflammatory, anticancer, and neuroprotective effects. Research shows butein protects against neurodegenerative diseases, cardiovascular issues, and metabolic disorders by promoting detoxification, modulating inflammation, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Butein and homobutein exhibit strong antioxidant and anti-tyrosinase activities.
Butein activates the Nrf2 pathway, enhancing cellular antioxidant defenses and modulating NF-κB signaling, exhibiting strong antioxidant bioactivity.
Butein activates Nrf2, protecting against oxidative stress, inflammation, obesity, glucose intolerance, insulin resistance, and offers anti-cancer and cardiovascular benefits.
Caffeic acid
Caffeic acid activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It offers anti-inflammatory, anticancer, and neuroprotective effects. Research shows caffeic acid protects against cardiovascular diseases, metabolic disorders, and neurodegenerative conditions by promoting detoxification, modulating inflammation, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Caffeic acid activates Nrf2, offering protection against oxidative stress, liver damage, fibrosis, inflammation, ischemia-reperfusion injury, allergic inflammation, and neurodegenerative diseases through its antioxidant properties.
Calendula officinalis
Calendula officinalis activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, wound-healing, and antimicrobial properties. Research shows Calendula officinalis protects against skin damage, promotes healing, and reduces inflammation in various conditions by inducing NRF2-mediated antioxidant gene expression and modulating inflammatory responses for cellular protection.
Calendula officinalis activates Nrf2, protecting against blood-brain barrier disruption, neuronal death, oxidative damage, and microglial overactivation. It supports astrocyte health and offers anti-inflammatory benefits.
Capsaicin
Capsaicin activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, analgesic, and anticancer properties. Research shows capsaicin protects against metabolic disorders, cardiovascular diseases, and neurodegenerative conditions by promoting detoxification, modulating inflammatory responses, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Capsaicin activates Nrf2, enhancing antioxidant enzymes to protect against contrast-associated acute kidney injury, ischemia/reperfusion-induced renal injury, cisplatin-induced nephrotoxicity, experimental diabetes-related renal injury, and chronic kidney disease progression.
Capsaicin activates PI3K-Nrf2 signaling, inducing heme oxygenase-1 expression in HepG2 cells.
Capsaicin induces heme oxygenase-1 in HepG2 cells by activating PI3K/Nrf2 signaling, increasing ROS production and enhancing Nrf2 nuclear translocation and ARE binding. It may inhibit NQO1 activity, contributing to ROS generation.
Capsaicin and sulforaphane prevent liver fibrosis by upregulating PPARγ and Nrf2.
Capsaicin and sulforaphane exhibit hepatoprotective effects by reducing liver fibrosis, downregulating profibrogenic genes, and enhancing PPARγ and Nrf2-mediated antioxidant activity in a murine model.
Carnosic acid
Carnosic acid activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits neuroprotective, anti-inflammatory, and anticancer effects. Research shows carnosic acid protects against neurodegenerative diseases, metabolic disorders, and oxidative damage by promoting detoxification, modulating inflammatory responses, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Carnosic acid activates Nrf2 by binding to Keap1, offering neuroprotection against oxidative stress and ischemia. It enhances antioxidant defenses, showing potential for treating neurodegenerative diseases like Parkinson’s and Alzheimer’s.
Carnosic acid activates Nrf2 and ATF4, inducing cytoprotective gene expression.
Carnosic acid activates Nrf2 and ATF4, enhancing nerve growth factor and antioxidant gene expression in an Nrf2-dependent and independent manner, revealing a novel gene regulation mechanism via cooperative activation.
Carnosol
Carnosol activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, neuroprotective, and anticancer properties. Research shows carnosol protects against neurodegenerative diseases, metabolic disorders, and cardiovascular issues by promoting detoxification, modulating inflammatory responses, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Carnosol activates Nrf2, providing anti-inflammatory and antioxidant benefits. It protects microvascular endothelial cells, improves endothelial barrier function, and may guard against diabetic microangiopathy and endothelial injury.
PB125® activates Nrf2, maintaining metabolic balance and redox homeostasis. It counteracts the decline in Nrf2 levels with aging, potentially reducing susceptibility to viral infections and normalizing dysregulated gene expression during pulmonary infections.
Carnosol inhibits oxidative stress and apoptosis in ovarian granulosa cells, protecting against polycystic ovary syndrome by activating Nrf2/HO-1 signaling through Keap1 interaction. It improves estrous cycles and reduces elevated androgen levels in PCOS mice.
Carotene
Carotene, a plant-derived carotenoid, activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, anticancer, and neuroprotective effects. Research shows carotene protects against chronic diseases such as cardiovascular issues, neurodegenerative disorders, and certain cancers by promoting detoxification, modulating inflammatory responses, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Lutein activates Nrf2 in human retinal pigment epithelial cells.
Carotenes like lutein activate Nrf2, enhancing antioxidant defenses. They protect against age-related macular degeneration, cardiovascular diseases, cancer, and oxidative stress-related inflammation.
Nrf2 activation plays a vital role in protecting against central nervous system trauma. Natural compounds that activate Nrf2 show neuroprotective effects in traumatic brain injury and spinal cord injury models, offering potential therapeutic strategies.
Catechin
Catechin, a type of flavonoid found in various foods, activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, anticancer, and neuroprotective effects. Research shows catechin protects against cardiovascular diseases, neurodegenerative disorders, and metabolic conditions by promoting detoxification, modulating inflammation, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Catechins, especially from green tea, activate Nrf2, protecting against cancer, cardiovascular diseases, neurodegenerative disorders, autoimmune diseases, and diabetes. They reduce oxidative stress and inflammation, promote gastrointestinal health, and offer antimicrobial and immunomodulatory benefits.
Catechins: Protective mechanism of antioxidant stress in atherosclerosis
Catechins enhance antioxidant activity by inhibiting ROS-producing enzymes and activating GSH, SOD, and Nrf2 pathways. They reduce oxidative stress by modulating Keap1/Nrf2/ARE and blocking NF-κB activation.
Chlorogenic Acid
Chlorogenic acid activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, anticancer, and neuroprotective properties. Research shows chlorogenic acid protects against chronic diseases such as cardiovascular disorders, diabetes, and neurodegenerative conditions by promoting detoxification, modulating inflammatory responses, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Chlorogenic acid activates Nrf2 via the WDR23-DDB1 pathway, inhibiting FOXO3 and enhancing antioxidant response. It extends C. elegans lifespan through the Akt-FOXO3/DAF16a-DDB1 axis, linking nutrient sensing and oxidative stress responses.
Chlorogenic acid activates Nrf2, enhancing antioxidant defenses and protecting against oxidative stress. It shows potential in preventing conditions like hepatitis B, aging, ARDS, cardiovascular diseases, neurodegenerative disorders, and various cancers. Additionally, it possesses anti-inflammatory, antiviral, and antimicrobial properties.
Cichoric acid
Cichoric acid activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, antiviral, and immunomodulatory properties. Research shows cichoric acid protects against chronic diseases such as diabetes, cardiovascular disorders, and neurodegenerative conditions by promoting detoxification, modulating inflammatory responses, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Cichoric acid prevents methotrexate-induced acute kidney injury by activating Nrf2/ARE/HO-1 signaling and inhibiting NF-κB/NLRP3 inflammasome activation, reducing oxidative stress and apoptosis.
Cichoric acid regulates hepatic glucose homeostasis and activates antioxidant responses, improving insulin resistance and hepatic injury in diabetes. It enhances AMPK phosphorylation, stimulates glycogen synthesis, and activates the Nrf2-Keap1 pathway for antioxidant enzyme expression.
Chicoric acid activates Nrf2, enhancing antioxidant enzymes and detoxification, reducing oxidative stress and inflammation. It protects against NAFLD, improves insulin sensitivity, inhibits NF-κB signaling, and modulates gut microbiota, showing therapeutic potential for metabolic dysfunction and related health issues.
CORIANDRUM SATIVUM
Coriandrum sativum, commonly known as coriander or cilantro, activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, antimicrobial, and potential anticancer properties. Research shows coriander protects against various chronic diseases, including metabolic disorders, cardiovascular issues, and gastrointestinal ailments, by promoting detoxification, modulating inflammatory responses, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Coriandrum sativum protects human keratinocytes from H2O2-induced oxidative stress by enhancing glutathione levels, increasing antioxidant enzyme activities, and activating Nrf2, demonstrating significant antioxidant effects.
Coriandrum sativum activates Nrf2, reducing oxidative stress and inflammation. It may protect against obesity, neurodegenerative diseases, cognitive decline, chronic inflammation, and age-related cellular senescence, supporting overall health.
Coriandrum sativum leaf extract activates Nrf2 via (E)-2-alkenals, reducing arsenic cytotoxicity. It modifies Keap1, upregulates phase-II enzymes, and enhances protection against inorganic arsenite, decreasing arsenic accumulation in liver tissue.
Crocin
Crocin, a carotenoid compound derived from saffron (Crocus sativus), activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, neuroprotective, and anticancer properties. Research shows crocin protects against various chronic diseases, including neurodegenerative disorders, cardiovascular issues, and metabolic syndromes, by promoting detoxification, modulating inflammatory responses, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Crocin activates the Nrf2 pathway, protecting against inflammation, oxidative stress, and cancer. Its antioxidant and therapeutic effects may benefit liver and heart health, provide neuroprotection, and offer chemoprotective properties.
Cigarette smoke exposure induces COPD in rats, leading to lung injury and cardiac dysfunction by decreasing Nrf2 and antioxidant expression. Crocin co-treatment restores these levels, highlighting Nrf2 activation as a therapeutic target for lung oxidative injuries.
Crocin and crocetin activate Nrf2, inducing antioxidant target genes for cellular protection.
Crocin and crocetin activate Nrf2 in HeLa cells, increasing luciferase activity and mRNA levels of HO-1, NQO1, and NQO2. Protein expression of NQO1 and HO-1 also increases, indicating enhanced antioxidant responses.
Curcumin
Curcumin, a bioactive compound found in turmeric (Curcuma longa), activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, anticancer, and neuroprotective properties. Research shows curcumin protects against various chronic diseases, including neurodegenerative disorders, cardiovascular issues, and metabolic syndrome, by promoting detoxification, modulating inflammatory responses, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Curcumin activates the Nrf2 pathway, providing cellular protection against oxidative injury.
Curcumin, a polyphenol from Curcuma longa, activates the Nrf2 pathway, enhancing antioxidant defenses and providing protection against oxidative damage. It exhibits therapeutic effects on neurodegenerative disorders, renal issues, and diabetes, making it a promising agent in cancer therapy. Curcumin stimulates Nrf2 through various mechanisms, including Keap1 inhibition and promoting Nrf2 nuclear translocation.
Curcumin activates the Nrf2 pathway, enhancing antioxidant enzyme expression and cellular protection against oxidative stress. It shows protective effects against acute kidney injury, liver damage, inflammatory disorders, and various toxins, indicating its potential therapeutic applications across multiple health conditions and diseases.
Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects
Nrf2 is a crucial transcription factor that regulates endogenous defenses against oxidative stress in the brain. It translocates to the nucleus, activating cytoprotective gene transcription through binding to antioxidant response elements (ARE). Curcumin and zinc–curcumin activate Nrf2, enhancing HO-1 levels and reducing its inhibitor, Keap1. This crosstalk improves cancer treatment responses and alleviates inflammation, while curcumin’s modulation of Nrf2 also aids in improving insulin resistance. Comprehensive studies support curcumin’s protective effects via Nrf2 regulation across various conditions.
Cyanidin-3-O-glucoside
Cyanidin-3-O-glucoside (C3G), an anthocyanin found in various fruits and vegetables, activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, neuroprotective, and anticancer properties. Research shows C3G protects against chronic diseases such as cardiovascular disorders, metabolic syndrome, and neurodegenerative conditions by promoting detoxification, modulating inflammatory responses, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Cyanidin-3-O-glucoside (C3G) protects HT22 cells from glutamate-induced oxidative toxicity by scavenging ROS and inhibiting intracellular ROS generation. It upregulates survival proteins like Nrf2 and ERK while activating endogenous antioxidant and phase II enzymes, suggesting C3G as a promising neuroprotectant against oxidative and ER stress.
Dandelion Extract
Dandelion extract, derived from the Taraxacum officinale plant, activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, hepatoprotective, and anticancer properties. Research shows dandelion extract protects against various chronic diseases, including liver damage, metabolic disorders, and inflammatory conditions, by promoting detoxification, modulating inflammatory responses, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Taraxacum officinale ethanol extract protects HT22 cells from glutamate-induced oxidative damage by activating the Nrf2/HO-1 pathways. It enhances cell viability, reduces ROS generation, and promotes Nrf2 nuclear translocation, highlighting its potential as a neuroprotective agent against neurodegenerative diseases.
Dandelion leaf and root extracts, along with taraxinic acid β-d-glucopyranosyl ester, activate Nrf2 in human hepatocytes, enhancing antioxidant activity. The extracts promote insulin release and inhibit alpha-glucosidase, demonstrating potential antihyperglycemic effects in metabolic syndrome treatment.
Danshen Extract
Danshen extract, derived from Salvia miltiorrhiza, activates the NRF2 pathway, enhancing antioxidant defenses and reducing oxidative stress. It exhibits anti-inflammatory, cardioprotective, and neuroprotective properties. Research shows Danshen extract protects against various chronic diseases, including cardiovascular disorders, neurodegenerative conditions, and diabetes, by promoting detoxification, modulating inflammatory responses, and inducing NRF2-mediated antioxidant gene expression for cellular protection.
Danshen modulates Nrf2 signaling, protecting against cisplatin-induced renal injury.
Danshen protects against cisplatin-induced renal dysfunction in mice by enhancing Nrf2 signaling. It reduces serum creatinine and blood urea nitrogen levels, mitigates kidney damage, and restores antioxidant enzyme activities. The findings suggest Danshen’s potential therapeutic role in preventing kidney injury through Nrf2 upregulation.
Review of Danshen covers metabolism and mechanisms behind its biological activities.
Danshensu exhibits neuroprotective effects in Parkinson’s models by activating the PI3K/AKT/Nrf2 pathway, enhancing GCLC, HO-1, and GCLM expression. Its antioxidant properties contribute to the therapeutic benefits of Danshen in preventing and treating various diseases.
Danshen induces HO-1 expression in RAW 264.7 macrophages via the PI3K/Akt-MEK1-Nrf2 pathway, reducing reactive oxygen species production. This highlights the cytoprotective role of HO-1 in Danshen’s antioxidant effects.
Dihydromyricetin
Cerebral ischemia‑reperfusion injury (CIRI) refers to the phenomenon that ischemic injury of the brain leads to the injury of brain cells, which is further aggravated after the recovery of blood reperfusion. Dihydromyricetin (DHM) has an effective therapeutic effect on vascular diseases; however, its role in CIRI has not been investigated. The oxygen and glucose deprivation/reoxygenation (OGD/R) cell model was used on HT22 hippocampal neurons in mice, by oxygen and sugar deprivation. DHM was found to increase the cell viability of HT22 cells following OGD/R induction. The levels of malondialdehyde (MDA) decreased, superoxide dismutase (SOD) and glutathione (GSH) in the OGD/R‑induced HT22 cells increased following DHM treatment, accompanied by the decreased protein expression levels of NOX2 and NOX4. DHM also inhibited cell apoptosis induced by OGD/R, and decreased the protein expression levels of Bax and caspase‑3, and increased the expression levels of Bcl‑2. Moreover, the expression levels of the NF‑E2‑related factor 2 (Nrf2)/heme oxygenase (HO‑1) signaling pathway‑associated proteins in OGD/R‑induced HT22 were increased following DHM treatment, and the effect of DHM on oxidative stress and apoptosis was reversed after the addition of the Nrf2/HO‑1 pathway inhibitor, brusatol. In conclusion, DHM inhibited oxidative stress and apoptosis in OGD/R‑induced HT22 cells by activating the Nrf2/HO‑1 signaling pathway.
Diosgenin
There was no obvious effect of diosgenin on the viability of ARPE‐19 cells and the viability of ARPE‐19 cells was significantly reduced after HG induction. However, diosgenin increased the viability, inhibited the apoptosis, and reduced the inflammatory response and oxidative stress of ARPE‐19 cells induced by HG. In addition, diosgenin could activate the AMPK/Nrf2/HO‐1 pathway. CC, an AMPK inhibitor, could reverse the above changes caused by diosgenin treatment in ARPE‐19 cells induced by HG.
Diosgenin could protect ARPE‐19 cells from inflammatory damage and oxidative stress induced by HG, by activating the AMPK/Nrf2/HO‐1 pathway.
Diosgenin decreased the blood glucose levels and increased the body weight of diabetic mice. There was a significant increase in the tail withdrawal latency of diabetic animals, and mechanical hyperalgesia was significantly alleviated after diosgenin treatment. Histopathological micrographs of HE-stained sciatic nerves showed improvement after diosgenin treatment. Diosgenin attenuated the level of MDA but increased the activities of SOD and GPx. Diosgenin increased the expression of Nrf2, HO-1 and NQO1.
Our results demonstrate that diosgenin can ameliorate behavioural and morphological changes in DPN by reducing oxidative stress. The Nrf2/HO-1 signalling pathway was involved in its neuroprotective effects.
Echinacea purpurea
Polysaccharide from Echinacea purpurea reduce the oxidant stress in vitro and in vivo
Echinacea purpurea polysaccharides (EPPS) were extracted through water extract and alcohol precipitate method. Three polysaccharides were purified by DEAE cellulose, named EPPS-1, EPPS-2 and EPPS-3. The antioxidant activities in three polysaccharides were screened by free radical scavenging test and EPPS-3 possessed the best antioxidant function. Then the antioxidant activities of EPPS-3 were further explored in oxidative damage model in vitro and in vivo for the first time. The results showed that the antioxidases and the metabolism content were significantly improved in EPPS-3 group. EPPS-3 could protect hepatic tissue from the injury of CCl4 compared with the oxidative damage model. The mechanism research demonstrated that EPPS-3 restrained cell apoptosis and promoted Nrf2 cell signal pathway to play an antioxidant impact. Therefore, EPPS-3 an ingredient could be served as amazing gift for food industry and feed additive.
Echinatin
Recent studies have shown that trimetazidine can delay the formation of age-related cataracts by regulating the expression of Nrf2 and reducing the production of ROS.53 Whitson et al.54 found that LECs lacking glutathione (GSH) depend on the activation of the Nrf2 signaling pathway to trigger oxidative stress. Moreover, Nrf2 inhibitors may increase the oxidative stress of the lens, and Nrf2 inducers can prevent cataract formation by reducing oxidative stress.55 Therefore, Nrf2 pathway activation can be used as a target for the prevention and treatment of age-related cataracts induced by oxidative stress.
In the present study, we found that Ech abolished the inhibitory effect of H2O2 on Nrf2 nuclear translocation in B3 cells, as well as the expression of HO -1 and NQO1. Furthermore, administration of the Nrf2 inhibitor ML385 could reverse the protective effect of Ech, suggesting that the potential antioxidant mechanism of Ech may include Nrf2 signal transduction. Importantly, it has been reported that Ech can inhibit activation of the NF-κB pathway56 and the AK T/mTOR p athway. 57 Therefore, the protective ef fect of Ech may also involve other signal pathways, a hypothesis that needs further study. In addition, because the potential toxicity and side effects of Ech and its derivatives are still unclear, there is still a lot of research to be performed before this drug can be applied in the clinic.
Eggplant extract
Effect of eggplant (Solanum melongena) on the metabolic syndrome: A review
Nrf2 is a transcription factor that binds to the ARE and thereby up-regulates the anti-oxidant gene expression such as superoxide dismutase (SOD) and heme oxygenase-1 (HO-1) (50). The protein kinase C (PKC) and Kelch-like ECH-associated protein 1 (Keap1) are intracellular redox sensors. Under basal conditions, Keap1 inhibits the Nrf2/ARE signaling pathway through direct interaction with Nrf2. Under oxidative stress conditions, elevated ROS interacts with cysteine residues of Keap1 and dissociate it from Nrf2. ROS also regulate PKC activity and result in the phosphorylation and activation of Nrf2. Then Nrf2 translocates from the cytoplasm into the nucleus and induces the expression of the anti-oxidant enzyme genes such as SOD, heme oxygenase-1 (HO-1), and NAD (P) H quinone oxidoreductase 1 (NOQ1) (51). The imbalance between ROS production and anti-oxidant enzyme expression leads to β-cell dysfunction and insulin resistance (51, 52). Therefore, the Nrf2 signaling pathway is able to act as a potential therapeutic target in diabetes. Another study has reported that purple eggplant contains anthocyanin compounds that exerted anti-oxidant properties.
The Role of the Nrf2/ARE Antioxidant System in Preventing Cardiovascular Diseases
It is widely believed that consuming foods and beverages that have high concentrations of antioxidants can prevent cardiovascular diseases and many types of cancer. As a result, many articles have been published that give the total antioxidant capacities of foods in vitro. However, many antioxidants behave quite differently in vivo. Some of them, such as resveratrol (in red wine) and epigallocatechin gallate or EGCG (in green tea) can activate the nuclear erythroid-2 like factor-2 (Nrf2) transcription factor. It is a master regulator of endogenous cellular defense mechanisms. Nrf2 controls the expression of many antioxidant and detoxification genes, by binding to antioxidant response elements (AREs) that are commonly found in the promoter region of antioxidant (and other) genes, and that control expression of those genes. The mechanisms by which Nrf2 relieves oxidative stress and limits cardiac injury as well as the progression to heart failure are described. Also, the ability of statins to induce Nrf2 in the heart, brain, lung, and liver is mentioned.
Elderberries extract anthocyanin
Sambucus nigra L. (Elderberry) is widely used as a dietary supplement in functional food and possesses many pharmacological activities to prevent ailments, such as the colds and fever, diabetes and cancer. However, research on its skin anti-aging effect is still limited. Here, we evaluated the recovery effects of elderberry extract (EB) in UVB-irradiated human skin keratinocytes (HaCaTs) and investigated whether EB represents a potential therapeutic agent against skin photoaging and inflammation. In this study, EB showed good efficiency on scavenging free radicals and dose-dependently reduced reactive oxygen species (ROS) generation. EB notably decreased UVB-induced matrix metalloproteinase-1 (MMP-1) expression and inflammatory cytokine secretion through the inhibition of mitogen-activated protein kinases/ activator protein 1 (MAPK/AP-1) and nuclear factor-κB (NF-κB) signaling pathways, blocking extracellular matrix (ECM) degradation and inflammation in UVB-irradiated HaCaTs. In addition, EB improved nuclear factor E2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) signaling to increase oxidative defense capacity, and enhanced transforming growth factor beta (TGF-β) signaling activation to promote procollagen type I synthesis, relieving UVB-induced skin cell damage. These results indicated that EB has the potential to ameliorate UVB-induced skin photoaging and inflammation.
Ellagic acid
Parkinson’s disease (PD) is the second most prevalent central nervous system (CNS) degenerative disease. Oxidative stress is one of key contributors to PD. Nuclear factor erythroid‐2‐related factor 2 (Nrf2) is considered to be a master regulator of many genes involved in anti‐oxidant stress to attenuate cell death. Therefore, activation of Nrf2 signalling provides an effective avenue to treat PD. Ellagic acid (EA), a natural polyphenolic contained in fruits and nuts, possesses amounts of pharmacological activities, such as anti‐oxidant stress and anti‐inflammation. Recent studies have confirmed EA could be used as a neuroprotective agent in neurodegenerative diseases. Here, mice subcutaneous injection of rotenone (ROT)‐induced DA neuronal damage was performed to investigate EA‐mediated neuroprotection. In addition, adult Nrf2 knockout mice and different cell cultures including MN9D‐enciched, MN9D‐BV‐2 and MN9D‐C6 cell co‐cultures were applied to explore the underlying mechanisms. Results demonstrated EA conferred neuroprotection against ROT‐induced DA neurotoxicity. activation of Nrf2 signalling was involved in EA‐mediated DA neuroprotection, as evidenced by the following observations. First, EA activated Nrf2 signalling in ROT‐induced DA neuronal damage. Second, EA generated neuroprotection with the presence of astroglia and silence of Nrf2 in astroglia abolished EA‐mediated neuroprotection. Third, EA failed to produce DA neuroprotection in Nrf2 knockout mice. In conclusion, this study identified EA protected against DA neuronal loss via an Nrf2‐dependent manner.
Parkinson’s disease (PD) is a familiar neurodegenerative disease, accompanied by motor retardation, static tremor, memory decline and dementia. Heredity, environment, age and oxidative stress have been suggested as key factors in the instigation of PD. The Keap1-Nrf2-ARE signaling is one of the most significant anti- oxidative stress (OS) pathways. The Keap1 is a negative regulator of the Nrf2. The Keap1-Nrf2-ARE pathway can induce cell oxidation resistance and reduce nerve injury to treat neurodegenerative diseases. Ellagic acid (EA) can inhibit the Keap1 to accumulate the Nrf2 in the nucleus, and act on the ARE to produce target proteins, which in turn may alleviate the impact of OS on neuronal cells of PD. This review analyzes the structure and physiological role of EA, along with the structure, composition and functions of the Keap1-Nrf2-ARE signaling pathway. We further expound on the mechanism of ellagic acid in its activation of the Keap1-Nrf2-ARE signaling pathway, as well as the relationship between EA in impairing the TLR4/Myd88/NF-κB and Nrf2 pathways. Ellagic acid has the potentiality of improving PD by activating the Keap1-Nrf2-ARE signaling pathway and scavenging free radicals.
The gastrointestinal tract is a key source of superoxide so as to be one of the most vulnerable to oxidative stress damage. Ellagic acid (EA), a polyphenol displays widely biological activities owing to its strong antioxidant properties. Here, we investigated the protective benefits of EA on oxidative stress and intestinal barrier injury in paraquet (PQ)-challenged piglets. A total of 40 weaned piglets were randomly divided into five groups: Control, PQ, 0.005% EA-PQ, 0.01% EA-PQ, and 0.02% EA-PQ. Piglets were intraperitoneally injected with 4 mg/kg (BW) PQ or saline on d-18, and sacrificed on d-21 of experiment. EA treatments eliminated growth-check induced by PQ and increased serum superoxide dismutase (SOD) activity but decreased serum malondialdehyde (MDA) level as compared to PQ group. EA supplementation promoted Nrf2 nuclear translocation and enhanced heme oxygenase-1 (HO-1) and quinone oxidoreductase 1 (NQO1) protein abundances of small intestinal mucosa. Additionally, EA improved PQ-induced crypt deepening, goblet cells loss, and villi morphological damage. Consistently, EA increased tight junction protein expression as was evident from the decreased serum diamine oxidase (DAO) levels. EA could ameliorate the PQ-induced oxidative stress and intestinal damage through mediating Nrf2 signaling pathway. Intake of EA-rich food might prevent oxidative stress-mediated gut diseases.
Epigallocatechin-3-gallate (EGCG)
The chemopreventive and chemoprotective activities of green tea have been attributed to the polyphenolic ingredient (-)-epigallocatechin-3-gallate (EGCG). Here, we report that treatment of human breast epithelial (MCF10A) cells with EGCG induces the expression of glutamate-cysteine ligase, manganese superoxide dismutase (MnSOD), and heme oxygenase-1 (HO-1). NF-E2-related factor (Nrf2) has been reported to regulate the antioxidant response element (ARE)-mediated expression of many antioxidant as well as detoxifying enzymes. The nuclear accumulation, ARE binding and transcriptional activity of Nrf2 were increased by EGCG treatment. Silencing of Nrf2 by siRNA gene knockdown rendered the MCF10A cells less sensitive to the EGCG-induced expression of HO-1 and MnSOD. Furthermore, EGCG activated Akt and extracellular signal-regulated protein kinase1/2 (ERK1/2). The pharmacologic inhibition of these kinases abrogated the nuclear translocation of Nrf2 induced by EGCG. These findings suggest that Nrf2 mediates EGCG-induced expression of some representative antioxidant enzymes, possibly via Akt and ERK1/2 signaling, which may provide the cells with acquired antioxidant defense capacity to survive the oxidative stress.
Epigallocatechin gallate upregulates Nrf2 to prevent diabetic nephropathy via disabling KEAP1
Epigallocatechin gallate (EGCG) is the most abundant and effective green tea catechin and has been reported to attenuate diabetic nephropathy (DN). However, the mechanism by which EGCG ameliorates DN, till now, has remained unclear. EGCG is known as a potent activator of nuclear factor erythroid 2-related factor 2 (Nrf2), which plays a key role in cellular defense against diabetes-induced oxidative stress and in the prevention of DN. In the present study, we tested whether Nrf2 is required for EGCG protection against DN. Therefore, C57BL/6 wild type (WT) and Nrf2 knockout mice were induced to diabetes by streptozotocin, in the presence or absence of a 24-week treatment with EGCG. In the WT mice, EGCG activated Nrf2 expression and function without altering the expression of Kelch-like ECH-associated protein 1 (Keap1). Diabetes-induced renal oxidative damage, inflammation, fibrosis and albuminuria were significantly prevented by EGCG. Notably, deletion of the Nrf2 gene completely abrogated these actions of EGCG. To further determine the effect of EGCG on KEAP1/Nrf2 signaling, mouse mesangial cells were treated with high glucose, in the presence of both Keap1 siRNA and EGCG. Interestingly, EGCG failed to enhance Nrf2 signaling and alleviate oxidative, inflammatory and fibrotic indicators, in the presence of Keap1 siRNA. The present study demonstrated, for the first time, that Nrf2 plays a critical role in EGCG protection against DN. Other findings indicated that in activation of KEAP1 protein by EGCG may mediate EGCG function in activating Nrf2.
Antioxidation Function of EGCG by Activating Nrf2/HO-1 Pathway in Mice with Coronary Heart Disease
The mice in the CHD model appeared to have myocardial pathological damage with elevated serum total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and decreased high-density lipoprotein cholesterol (HDL-C). Of note, administration of EGCG significantly attenuated myocardial injuries and improved blood lipid levels in mice in a concentration-dependent manner. The advent of EGCG significantly decreased the expression of VEGFA and MMP-2 and increased the activity of superoxide dismutase (SOD), when reducing the content of reactive oxygen species (ROS) in the myocardial tissue and upregulating the expression of HO-1, NQO1, and Nrf2.
EGCG may reduce atherosclerotic plaque and alleviate pathological damage in the cardiac tissue of CHD mice as well as improve blood lipid levels with antioxidative effect. The mechanism of its effect may be related to the activation of the Nrf2/HO-1/NQO1 antioxidant pathway in vivo of the CHD mice.
Ethyl ferulate
These findings suggest that ethyl ferulate ameliorated hyperglycemia-induced oxidative stress by increasing renal Nrf2 levels, thereby preventing diabetes-induced kidney injury. In conclusion, the present study endorses the usefulness of Nrf2 activators, such as ethyl ferulate, as adjuvant therapy for preventing the diabetic nephropathy.
Ethyl ferulate (ethyl-3-hydroxyl-4-methoxycinnamate), a phenylpropanoid, is a naturally occurring ethyl ester of ferulic acid and is widely present in plants and especially grains, such as rice and maize. Our study has highlighted the renoprotective effect of ethyl ferulate in preventing diabetes-associated renal injury. The observed effect of ethyl ferulate is due to amelioration of diabetes-induced oxidative stress and inflammation, by activation of the Nrf2 pathway. These results indicate the potential of ethyl ferulate as a nutraceutical or adjuvant therapy in prevention of diabetic nephropathy.
Eupatolide
TTIJ notably attenuated LPS-induced histopathological changes of lung. The RNA-seq result suggested that the protective effect of TTIJ on LPS-induced ALI were associated with the Toll-like receptor 4 (TLR4) and nuclear factor-erythroid 2-related factor 2 (Nrf2) signaling pathways. Pretreatment with TTIJ significantly reduced the inflammation and oxidative stress via regulating levels of pro-inflammatory and anti-oxidative cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), superoxide dismutase (SOD), and glutathione (GSH), in LPS-induced ALI mice. TTIJ treatment could suppress the cyclooxygenase-2 (COX-2) expression level and the phosphorylation of p65, p38, ERK, and JNK through the in activation of the MAPK/NF-κB signaling pathway in a TLR4-independent manner. Meanwhile, TTIJ treatment upregulated expression levels of proteins involved in the Nrf2 signaling pathway, such as heme oxygenase-1 (HO-1), NAD(P)H: quinoneoxidoreductase-1 (NQO-1), glutamate-cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase modifier subunit (GCLM), via activating the Nrf2 receptor, which was confirmed by the luciferase assay.
TTIJ could activate the Nrf2 receptor to alleviate the inflammatory response and oxidative stress in LPS-induced ALI mice, which suggested that TTIJ could serve as the potential agent in the treatment of ALI.
Key findings: TEIJ significantly alleviated the course of ALI via suppressing the interstitial infiltrated inflammatory cells, the increase of inflammatory factors and the decrease of anti-oxidative factors. TEIJ inactivated the MAPK/NF-κB signalling pathway to suppress the transcription of its downstream target genes, such as TNF-α, IL-6, etc. Meanwhile, TEIJ activated the Keap1/Nrf2 signalling pathway to regulate expression levels of Nrf2 and its target proteins. The results of LC-QTOF-MS/MS indicated potential active constituents of I. japonica, terpenoids and flavonoids. Additionally, terpenoids and flavonoids synergistically alleviated LPS-induced ALI depending on MAPK/NF-κB and Keap1/Nrf2 signalling pathways.
Conclusion: I. japonica could be considered a potential agent to treat ALI via regulating the MAPK/NF-κB and Keap1/Nrf2 signaling pathways.
Ferulic Acid
Ferulic acid ameliorates neurodegeneration via the Nrf2/ARE signalling pathway
It has been suggested that inhibition of these enzyme may prevent the changes triggered by oxidative stress. FA also interacts with enzymes like heme oxygenase1, succinate dehydrogenase, biliverdin reductase, heat shock protein 7 and catalase that play vital role in MAPK and Nrf2 signaling pathways by scavenging the ROS/RNS thus preventing cell death [37]. In research for AD, it is observed that ferulic acid ethyl ester scavenges free radical by decreasing the fluorescence intensity in DCF assay in gerbils treated with Fe2+/H2O2 or AAPH. Thus showing that ester of ferulic acid overcomes oxidative stress by reducing intracellular ROS [38]. FA also improves cell viability and reduces ROS in PC-12 cells treated with 6-OHDA or H2O2 in Parkinson’s disease model and also induces autophagy reducing the ROS accumulation preventing cell death [39].
Gallic acid
Gallic Acid Alleviates Gouty Arthritis by Inhibiting NLRP3 Inflammasome activation and Pyroptosis Through Enhancing Nrf2 signaling
Gallic acid is an active phenolic acid widely distributed in plants, and there is compelling evidence to prove its anti-inflammatory effects. NLRP3 inflammasome dysregulation is closely linked to many inflammatory diseases. However, how gallic acid affects the NLRP3 inflammasome remains unclear. Therefore, in the present study, we investigated the mechanisms underlying the effects of gallic acid on the NLRP3 inflammasome and pyroptosis, as well as its effect on gouty arthritis in mice. The results showed that gallic acid inhibited lactate dehydrogenase (LDH) release and pyroptosis in lipopolysaccharide (LPS)-primed and ATP-, nigericin-, or monosodium urate (MSU) crystal-stimulated macrophages. Additionally, gallic acid blocked NLRP3 inflammasome activation and inhibited the subsequent activation of caspase-1 and secretion of IL-1β. Gallic acid exerted its inhibitory effect by blocking NLRP3-NEK7 interaction and ASC oligomerization, thereby limiting inflammasome assembly.
Moreover, gallic acid promoted the expression of nuclear factor E2-related factor 2 (Nrf2) and reduced the production of mitochondrial ROS (mtROS). Importantly, the inhibitory effect of gallic acid could be reversed by treatment with the Nrf2 inhibitor ML385. Nrf2 siRNA also abolished the inhibitory effect of gallic acid on IL-1β secretion. The results further showed that gallic acid could mitigate MSU-induced joint swelling and inhibit IL-1β and caspase 1 (p20) production in mice. Moreover, gallic acid could moderate MSU-induced macrophages and neutrophils migration into joint synovitis. In summary, we found that gallic acid suppresses ROS generation, thereby limiting NLRP3 inflammation activation and pyroptosis dependent on Nrf2 signaling, suggesting that gallic acid possesses therapeutic potential for the treatment of gouty arthritis.
Environmental pollution is one of the risk factors for respiratory diseases. The nuclear factor erythroid 2-related factor 2 (Nrf2) is the major mechanisms contributing to cellular defense against oxidative damage. Gallic acid (GA) is regarded as potent anti-inflammatory and antioxidant agents. The aim was to evaluate the role of Nrf2 pathway in particulate matter (PM10) exposure on lung and epithelial cells with an emphasis on the role of GA. In in vivo part, the rats were divided as control, GA (30 mg/kg), particulate matter (PM) (0.5, 2.5, and 5 mg/kg), and PM + GA. In in vitro study, the cells were divided as control, PM10 (100, 250, and 500 µg/ml), GA (50 µmol/L) and PM10+GA.
Inflammation, oxidative stress and Nrf2-pathway factors were assessed. PM10 groups showed a considerable increase in the epithelial permeability and inflammatory parameters. We also found a significant decrease in the expression of Nrf2 and its up-stream regulators genes. Accordingly, the biosynthesis of glutathione (GSH) and other antioxidant activities significantly decreased. Gallic acid was identified to restore the antioxidant status to the normal levels. Our findings approved that Nrf2 is involved in PM10-induced oxidative damages and showed that Nrf2 activation by natural agents could ameliorate respiratory injuries induced by PM10.
Gallic acid (GA), a natural polyphenol, has been shown to exert a variety of heath promoting effects. We herein investigated the critical role of nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated antioxidant response in the protection of GA against tert-butyl hydroperoxide (t-BHP)-induced hepatotoxicity in L02 cells. Pretreatment of GA prevented the hepatocytotoxicity induced by t-BHP, as evidenced by the facts that GA suppressed t-BHP-induced cytotoxicity and reactive oxygen species (ROS) generation. GA induced nuclear translocation of Nrf2 along with expression of target proteins, including heme oxygenase-1 (HO-1) and glutamate cysteine ligase catalytic modify subunit (GCLC), and increased intracellular glutathione (GSH) content. Additionally, GA induced phosphorylated activation of extracellular regulated kinase (ERK), and ERK inhibitor PD98059 partially decreased GA-induced hepatoprotection, and downregulated the increased protein expressions of Nrf2, GCLC and HO-1 induced by GA. Interestingly, we found that GA could enhance the thermal stability of Keap1, which indicated the potential interaction between GA and Keap1. Furthermore, molecular docking indicated that GA possibly competed with Nrf2 for binding to Keap1. Collectively, GA effectively protects against t-BHP-induced hepatotoxicity via inducing ERK/Nrf2-mediated antioxidative signaling pathway. Meanwhile, GA disturbs protein-protein interaction between Keap1 and Nrf2 which might also contribute to nuclear translocation of Nrf2.
Ganodermanondiol
Ganodermanondiol, a biologically active compound, was isolated from the Lingzhi mushroom (Ganoderma lucidum). The present study examined the protective effects of ganodermanondiol against tert-butyl hydroperoxide (t-BHP)-induced hepatotoxicity. Ganodermanondiol protected human liver-derived HepG2 cells through nuclear factor-E2-related factor 2 (Nrf2) pathway-dependent heme oxygenase-1 expressions. Moreover, ganodermanondiol increased cellular glutathione levels and the expression of the glutamine-cysteine ligase gene in a dose-dependent manner. Furthermore, ganodermanondiol exposure enhanced the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) and its upstream kinase activators, LKB1 and Ca(2+)/calmodulin-dependent protein kinase-II (CaMKII).
This study indicates that ganodermanondiol exhibits potent cytoprotective effects on t-BHP-induced hepatotoxicity in human liver-derived HepG2 cells, presumably through Nrf2-mediated antioxidant enzymes and AMPK.
Garcinone D
Results showed that GE pretreating noticeably diminishes both the serum indices (transaminases, ALP, LDH, and γ-GT) and histopathological lesions of the liver. It counteracted neutrophil and CD4+ infiltration into the liver. GE furthered the Nrf2 genetic expression and its antioxidants’ cascade, which resulted in amelioration of Con-A-caused oxidative stress (OS), lipid per-oxidative markers (4-HNE, MDA, PC) reduction, and intensified antioxidants (TAC, SOD, GSH) in the hepatic tissue. Additionally, GE prohibited NF-ĸB (nuclear factor kappa-B) activation and lessened the genetics and levels of downstream cytokines (IL1β and IL6). Moreover, the TNF-α/JNK axis was repressed in GE-treated mice, which was accompanied by attenuation of Con-A-induced apoptosis. These findings demonstrated the protective potential of GE in Con-A-induced hepatitis which may be associated with Nrf2/HO-1 signaling activation and OS suppression, as well as modulation of the NF-κB and TNF-α/JNK/apoptosis signaling pathway. These results suggest the potential use of GE as a novel hepato-protective agent against autoimmune hepatitis.
Xanthones from the tropical fruit mangosteen (Garcinia mangostana) display anti-inflammatory and anti-oxidative activities. Here, we isolate and identify potential inducers of the aryl hydrocarbon receptor (AhR) and nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathways from mangosteen using a bioassay-guided strategy. Mangosteen fruit pericarp extracts were subjected to sequential solvent extractions, followed by chromatography coupled with NMR spectroscopy and mass spectrometric analyses for identification and isolation of pure compounds. Isolation of active fractions led to seven prenylated xanthones that were identified and subsequently evaluated for bioactivity. In vitro luciferase reporter cellular assays using H1L6.1c3 (AhR induction) and HepG2-ARE (Nrf2 induction) were used to identify AhR and Nrf2 activators. All seven prenylated xanthones displayed AhR inducing activity, whereas only five xanthones activated Nrf2. Garcinone D (GarD) significantly upregulated AhR/Cyp1a1 and Nrf2/HO-1 protein expression and enhanced zonula occludens-1 and occludin protein levels in HT-29 cells. In addition, GarD inhibited oxidative stress-induced intestinal epithelial barrier dysfunction by enhancing tight junction (TJ) proteins and inhibition of reactive oxygen species production. Inhibition of AhR by pretreating cells with an AhR antagonist revealed that the AhR pathway is required for the improved epithelial barrier functions of GarD. These results highlight a dual mechanism by GarD that confers protection against intestinal epithelial barrier dysfunction.
Genistein
Cancer is one of the leading causes of death worldwide. Chemotherapy and radiation therapy are currently providing the basis for cancer therapies, although both are associated with significant side effects. Thus, cancer prevention through dietary modifications has been receiving growing interest. The potential of selected flavonoids in reducing carcinogen-induced reactive oxygen species (ROS) and DNA damage through the activation of nuclear factor erythroid 2 p45 (NF-E2)-related factor (Nrf2)/antioxidant response element (ARE) pathway was studied in vitro. Dose-dependent effects of pre-incubated flavonoids on pro-carcinogen 4-[(acetoxymethyl)nitrosamino]-1-(3-pyridyl)-1-butanone (NNKAc)-induced ROS and DNA damage in human bronchial epithelial cells were studied in comparison to non-flavonoids.
The most effective flavonoids were assessed for the activation of Nrf2/ARE pathway. Genistein, procyanidin B2 (PCB2), and quercetin significantly suppressed the NNKAc-induced ROS and DNA damage. Quercetin significantly upregulated the phosphorylated protein kinase B/Akt. PCB2 significantly upregulated the activation of Nrf2 and Akt through phosphorylation. Genistein and PCB2 significantly upregulated the phospho-Nrf2 nuclear translocation and catalase activity. In summary, genistein and PCB2 reduced the NNKAc-induced ROS and DNA damage through the activation of Nrf2. Further studies are required to understand the role of dietary flavonoids on the regulation of the Nrf2/ARE pathway in relation to carcinogenesis.
Results: Our results showed that genistein treatment effectively reduced cerebral infarction, attenuated neuronal injury and apoptosis, and contributed to the long-term recovery of neurological outcomes and brain atrophy in neonatal HIBD mice. Moreover, genistein ameliorated HIBD-induced oxidative stress and neuroinflammation. Meanwhile, genistein significantly increased cell viability, reversed neuronal injury and decreased cell apoptosis after OGD/R injury. Finally, the activation of the Nrf2/HO-1 pathway and inhibition of the NF-κB pathway by genistein were verified in the brain tissues of neonatal mice subjected to HIBD and in primary cortical neurons exposed to OGD/R.
Conclusions: Genistein exerted neuroprotective effects on HIBD by attenuating oxidative stress and neuroinflammation through the Nrf2/HO-1 and NF-κB signalling pathways.
In this study, we focused on the concerted effects on expression of Nrf2 and phase II enzyme pathway components. Transient transfection assays, RT-PCR and immunoblot analysis were performed to study its molecular mechanisms of action. In Caco-2 cells, treatment with genistein markedly attenuated H2O2-induced peroxide formation; this amelioration was reversed by buthionine sulfoximine (GCLC inhibitor) and zinc protoporphyrin(HO-1 inhibitor). Genistein increased HO-1 and GCLC mRNA and protein expression. Genistein treatme nt activated the ERK1/2 and PKC signaling pathway; therefore increased Nrf2 mRNA and protein expression. The roles of the ERK1/2 and PKC signaling pathway were determined using PD98059 (ERK1/2 inhibitor) and GF109203X (PKC inhibitor) and RNA interference directed against Nrf2. Both inhibitors and siNrf2 abolished genistein-induced HO-1 and GCLC protein expression. These results suggest the involvement of ERK1/2, PKC, and Nrf2 in inducing HO-1 and GCLC by genistein.
Our studies show that genistein up-regulated HO-1 and GCLC expression through the EKR1/2 and PKC /Nrf2 pathways during oxidative stress.
Ginkgo leaves Flavone Glycoside
Objectives: This review summarises the current findings regarding the therapeutic effects of GBE and its active ingredients in relation to the Nrf2 antioxidant cascade, to provide scientific insights into the clinical applications of GBE in treating oxidative stress-induced diseases.
Key findings: We found that GBE or its active ingredients activate several signalling mechanisms in cells, including the Nrf2 pathway, which is the master controller of the antioxidant defence that detoxifies reactive oxygen species (ROS). ROS-mediated cell and tissue damage contributes to ageing and pathological conditions that underlie several important human diseases, such as diabetic nephropathy (DN), ischemic stroke and age-related macular degeneration (AMD).
Summary: GBE or its component antioxidants could be applied for the treatment and/or prevention of DN, ischemic stroke and AMD due to their capacity to activate Nrf2 signalling. These strategies may also be applicable to the treatment of other similar conditions that are induced by oxidative stress. Thus, the therapeutic applications of GBE could be expanded.
Objectives: Age-related macular degeneration (AMD) is a prevalent ocular disease. Dry AMD accounts for most cases of blindness associated with AMD but there are no treatments. oxidative stress-induced damage to retinal pigment epithelial (RPE) cells is a major contributor to the pathogenesis of dry AMD. This study investigated the protective actions of Ginkgo biloba extracts (GBE) in human RPE cells subjected to tert-butyl hydroperoxide (t-BHP)-mediated oxidative stress.
Methods: The human ARPE-19 cells were pre-treated with or without GBE before the exposure to t-BHP. Cell viability, cell death profile and lipid peroxidation were assessed. The findings were verified using human primary RPE cultures.
Key findings: GBE pre-treatment prevented the increase in lipid peroxidation and necrosis/ferroptosis, and the concurrent viability decrease in RPE cells exposed to t-BHP. It enabled the pronounced activation of Nrf2 and its downstream genes. We found that ERK1/2 phosphorylation was increased to a similar extent by t-BHP and GBE.
Conclusion: This study revealed that GBE pre-treatment attenuates pro-oxidant stress and protects human RPE cells from oxidative injury by modulating ERK1/2-Nrf2 axis. These findings suggest that GBE has the potential to be developed as a agent that may be valuable in decreasing AMD progression.
Vitiligo is a common skin depigmenting disorder characterized by the loss of functional melanocytes. Its pathogenesis is complicated and oxidative stress plays a critical role in the development of vitiligo. Thus, antioxidant therapy is a promising therapeutic strategy to prevent or even reverse the progression of depigmentation. Ginkgo biloba extract EGb761 has been confirmed to have protective effects on neurons against oxidative stress. Notably, several clinical trials have shown that patients with stable vitiligo achieved repigmentation after taking EGb761. However, the exact mechanism underlying the protective effects of EGb761 on melanocytes against oxidative stress has not been fully elucidated. In the present study, we found that EGb761 effectively protected melanocytes against oxidative stress-induced apoptosis and alleviated the excessive accumulation of reactive oxygen species (ROS) and lipid peroxidation by enhancing the activity of antioxidative enzymes. Furthermore, the antioxidative effect of EGb761 was achieved by activating Nrf2 and its downstream antioxidative genes. In addition, interfering Nrf2 with siRNA abolished the protective effects of EGb761 on melanocytes against oxidative damage. In conclusion, our study proves that EGb761 could protect melanocytes from H2 O2 -induced oxidative stress by activating Nrf2. Therefore, EGb761 is supposed to be a potential therapeutic agent for vitiligo.
Glucoraphanin
As the prevalence of inflammatory bowel diseases (IBD) rises, the etiology of IBD draws increasing attention. Glucoraphanin (GRP), enriched in cruciferous vegetables, is a precursor of sulforaphane, known to have anti-inflammatory and antioxidative effects. We hypothesized that dietary GRP supplementation can prevent mitochondrial dysfunction and oxidative stress in an acute colitis mouse model induced by dextran sulfate sodium (DSS). Eight-week-old mice were fed a regular rodent diet either supplemented with or without GRP. After 4 weeks of dietary treatments, half of the mice within each dietary group were subjected to 2.5% DSS treatment to induce colitis. Dietary GRP decreased DSS-induced body weight loss, disease activity index, and colon shortening. Glucoraphanin supplementation protected the colonic histological structure, suppressed inflammatory cytokines, interleukin (IL)-1β, IL-18, and tumor necrosis factor-α (TNF-α), and reduced macrophage infiltration in colonic tissues. Consistently, dietary GRP activated AMP-activated protein kinase (AMPK), peroxisome proliferator-activated receptor-gamma co activator (PGC)-1α, and nuclear factor erythroid 2-related factor 2 (Nrf2) pathways in the colonic tissues of DSS-treated mice, which was associated with increased mitochondrial DNA and decreased content of the oxidative product 8-hydroxydeoxyguanosine (8-OHDG), a nucleotide oxidative product of DNA. In conclusion, dietary GRP attenuated mitochondrial dysfunction, inflammatory response, and oxidative stress induced by DSS, suggesting that dietary GRP provides a dietary strategy to alleviate IBD symptoms.
Non-alcoholic fatty liver disease (NAFLD) is a common disease in Western nations and ranges in severity from steatosis to steatohepatitis (NASH). NAFLD is a genetic-environmental-metabolic stress-related disease of unclear pathogenesis. NAFLD is triggered by caloric overconsumption and physical inactivity, which lead to insulin resistance and oxidative stress. A growing body of evidence indicates that mitochondrial dysfunction plays a critical role in the pathogenesis of NAFLD. Mitochondrial dysfunction not only promotes fat accumulation, but also leads to generation of reactive oxygen species (ROS) and lipid peroxidation, resulting in oxidative stress in hepatocytes. Nuclear factor erythroid 2-related factor 2 (Nrf2) is an important modulator of antioxidant signaling that serves as a primary cellular defense against the cytotoxic effects of oxidative stress. The pharmacological induction of Nrf2 ameliorates obesity-associated insulin resistance and NAFLD in a mouse model. Sulforaphane and its precursor glucoraphanin are derived from broccoli sprouts and are the most potent natural Nrf2 inducers-they may protect mitochondrial function, thus suppressing the development of NASH. In this review, we briefly describe the role of mitochondrial dysfunction in the pathogenesis of NASH and the effects of glucoraphanin on its development.
Glutathione
Nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) is the primary transcription factor protecting cells from oxidative stress by regulating cytoprotective genes, including the antioxidant glutathione (GSH) pathway. GSH maintains cellular redox status and affects redox signaling, cell proliferation, and death. GSH homeostasis is regulated by de novo synthesis as well as GSH redox state; previous studies have demonstrated that Nrf2 regulates GSH homeostasis by affecting de novo synthesis. We report that Nrf2 modulates the GSH redox state by regulating glutathione reductase (GSR). In response to oxidants, lungs and embryonic fibroblasts (MEFs) from Nrf2-deficient (Nrf2−/−) mice showed lower levels of GSR mRNA, protein, and enzyme activity relative to wild type (Nrf2+/+). Nrf2−/− MEFs exhibited greater accumulation of glutathione disulfide and cytotoxicity compared to Nrf2+/+ MEFs in response to t-butylhydroquinone, which was rescued by restoring GSR. Microinjection of glutathione disulfide induced greater apoptosis in Nrf2−/− MEFs compared to Nrf2+/+ MEFs. In silico promoter analysis of the GSR gene revealed three putative antioxidant-response elements (ARE1, −44; ARE2, −813; ARE3, −1041). Reporter analysis, site-directed mutagenesis, and chromatin immunoprecipitation assays demonstrated binding of Nrf2 to two AREs distal to the transcription start site. Overall, Nrf2 is critical for maintaining the GSH redox state via transcriptional regulation of GSR and protecting cells against oxidative stress.
The level of glutathione (GSH) is increased in many cancer cells. Consuming intracellular GSH by chemical small molecules that specifically target GSH is a new strategy to treat cancer. Recently, we synthesized and proved that a new compound 2-(7-(diethylamino)-2-oxo-2H-chromen-3-yl)cyclohexa-2,5-diene-1,4-dione (PBQC) could target to and consume intracellular GSH specifically, but, it is not clear if PBQC can affect cancer cell growth and the activity of the nuclear factor-erythroid 2-related factor 2 (Nrf2) which is a key factor involved in regulation of cancer cell growth. In this study, we addressed these questions. We found that PBQC suppressed cancer cell growth through increasing the activity of Nrf2, while it did not inhibit normal vascular endothelial cell growth. Furthermore, we demonstrated that PBQC can cause Keap-1 protein S-glutathionylation and promote Nrf2 nuclear translocation as well as the expression of pro-apoptosis genes. As a result, the cancer cells underwent apoptosis. Here, we provide a new Nrf2 activator, PBQC that can promote the expressions of pro-apoptosis genes downstream Nrf2. The data suggest that PBQC is a potential lead-compound for development of new anti-cancer drugs.
Glycyrrhizinic Acid
Effects of glycyrrhizin on the expression of phosphorylation of AKT, Nrf2, and HO‐1 in ARPE‐19 cells treated with sodium iodate (SI) by Western blot detection. A, Effects of glycyrrhizin on phosphorylation of AKT. B, Glycyrrhizin significantly increased the phosphorylation of AKT at a concentrations of 25‐200 μmol (**P < 0.05 vs the SI control, *P < 0.01 vs the SI control). C, Effects of glycyrrhizin on the expression of Nrf2. D, Glycyrrhizin significantly increased the expression of Nrf2 at concentrations of 25‐200 μmol (*P < 0.01 vs the SI control). E, Effects of glycyrrhizin on the expression of HO‐1. F, Glycyrrhizin significantly increased the expression of HO‐1 at concentrations of 25‐200 μmol (*P < 0.01 vs the SI control). G, Effects of PI3K/AKT inhibitor LY294002 on phosphorylation of AKT treated with glycyrrhizin.
H, Glycyrrhizin significantly increased the phosphorylation of AKT at a concentrations of 50 μmol (# P < 0.01 vs the SI control), while LY294002 significantly decreased the phosphorylation of AKT (*P < 0.01 vs the SI + GA50 control). I, Effects of LY294002 on the expression of Nrf2. J, Glycyrrhizin significantly increased the expression of Nrf2 at concentrations of 50 μmol (#P < 0.01 vs the SI control), while LY294002 significantly decreased the expression of Nrf2 (*P < 0.01 vs the SI + GA50 control). K, Effects of glycyrrhizin on the expression of HO‐1. L, Glycyrrhizin significantly increased the expression of HO‐1 at concentrations of 50 μmol (# P < 0.01 vs the SI control), while LY294002 significantly decreased the expression of HO‐1(*P < 0.01 vs the SI + GA50 control)
Our findings demonstrated that GA alleviated RTS-induced elevation of serum ALT and bilirubin levels, as well as hepatocytes necrosis and sinusoidal endothelial cells (SECs) damage in rats. GA also enhanced the activities and expressions of several antioxidant enzymes through upregulating nuclear factor-erythroid 2-related factor2 (Nrf2). Moreover, inhibition of Nrf2 blocked the hepatoprotective effect of GA against RTS intoxication. Mechanistically, GA increased the phosphorylation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) and enhanced glycogen synthase kinase 3 beta (GSK3β) inhibitory phosphorylation at serine 9, thus promoting the nuclear accumulation of Nrf2 and activating its downstream targets.
Conclusion
This study for the first time demonstrated that GA exerted protective effects against RTS-induced liver injury by potentiating the Nrf2-mediated antioxidant system through PI3K/Akt/GSK3β pathway. The findings indicated that GA may serve as a potential candidate drug for the treatment of PA intoxication.
18α–Glycyrrhetinic acid (18α-GA) is a bioactive triterpenoid that has been shown to activate the nuclear factor (erythroid-derived-2)-like 2 (Nrf2), the main transcription factor that orchestrates the cellular antioxidant response, in both cellular and organismal context. Although various beneficial properties of 18α-GA have been revealed, including its anti-oxidation and anti-aging activity, its possible protective effect against DNA damage has never been addressed. In this study, we investigated the potential beneficial properties of 18α-GA against DNA damage induced by mitomycin C (MMC) treatment. Using human primary fibroblasts exposed to MMC following pre-treatment with 18α-GA, we reveal an Nrf2-mediated protective effect against MMC-induced cell death that depends on extracellular signal–regulated kinase (ERK) signaling. In total, our results reveal an additional beneficial effect of the Nrf2 activator 18α-GA, suggesting that this important phytochemical compound is a potential candidate in preventive and/or therapeutic schemes against conditions (such as aging) or diseases that are characterized by both oxidative stress and DNA damage.
Goniothalamin
Goniothalamin (GTN), a styryl-lactone, is a secondary metabolite naturally found in its enantiomeric form (R) in plants of the genus Goniothalamus (Annonaceae). The antiproliferative activity against human tumor cell lines reported in several studies suggest that the α,β-unsaturated δ-lactone moiety emerges as a key Michael acceptor for cysteine residues or other nucleophilic biological molecules. Herein we describe the gastroprotective activity of rac-GTN on chemical-induced gastric ulcers models in rats. GTN has a potent gastroprotective effect on ethanol-induced ulcers (effective dose50 = 18 mg/kg) and this activity is dependent on sulfhydryl compounds and prostaglandins generation, but independent of nitric oxide (NO), gastric secretion and mucus production. We hypothesize that goniothalamin may act as a mild irritant, inducing the production of sulfhydryl compounds and prostaglandins, in a process known as adaptive cytoprotection. This hypothesis is supported by the fact that Michael acceptors are the most potent inducers of antioxidant response (as activation of Nrf2 pathway) through generation of mild oxidative stress and that gastroprotective activity of goniothalamin is inhibited after pre-treatment with NEM (N-ethylmaleimide) and NSAID (non-steroidal anti-inflammatory drugs), highlighting the importance of sulfhydryl compounds and prostaglandins on GTN activity.
Grape seed procyanidins
Results: Our results proved that combined ethanolic extract of Vitis vinifera and Silymarin restored normal renal and cardiac histomorphology. Significant improvement of Creatinine, BUN, lipid profile and hs-CRP cardiac and renal biochemical indicators confirmed our results. Moreover, significant elevation of mRNA expression levels of Nrf2 proved that combined Vitis vinifera and Silymarin action was directly related to the redox-sensitive regulator pathway.
Conclusions: We concluded that synergistic therapeutic effect of Vitis vinifera extract and Silymarin on experimental cardiorenal injury model owes principally to promoting activation of the Keap1/Nrf2 signaling pathway.
Green Tea Polyphenols
Methods and results: Nrf2-null and wild-type (WT) mice were fed an HF diet containing 0 or 2% GTE for eight weeks prior to assessing parameters of NASH. Compared to WT mice, Nrf2-null mice had increased serum alanine aminotransferase, hepatic triglyceride, expression of free fatty acid uptake and lipogenic genes, malondialdehyde and NFκB phosphorylation and expression of pro-inflammatory genes. In WT mice, GTE increased Nrf2 and NADPH:quinone oxidoreductase-1 mRNA, and lowered hepatic steatosis, lipid uptake and lipogenic gene expression, malondialdehyde, and NFκB-dependent inflammation. In Nrf2-null mice, GTE lowered NFκB phosphorylation and TNF-α and MCP1 mRNA to levels observed in WT mice fed GTE whereas hepatic triglyceride and lipogenic genes were lowered only to those of WT mice fed no GTE. Malondialdehyde was lowered in Nrf2-null mice fed GTE, but not to levels of WT mice, and without improving the hepatic antioxidants α-tocopherol, ascorbic acid and uric acid.
Conclusion: Nrf2 deficiency exacerbates NASH whereas anti-inflammatory and hypolipidemic activities of GTE likely occur largely independent of Nrf2 signaling.
In aerobic conditions, the transfer of electrons occurs between atoms, wherein oxygen is the ultimate electron acceptor which produces ATP [20]. However, the transfer of uncoupled electrons results in the generation of free radicals such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) [21]. ROS are produced regularly inside the body, specifically in the mitochondria, during respiration and other immune-related functions [22,23]. They can act as a mobile signaling messenger inside the host. The overall cellular health is dependent on the level of ROS inside the host [24]. The endoplasmic reticulum transmembrane protein (IRE-1) mediates homeostasis by initializing the unfolded protein response pathway. However, during the accumulation of ROS, IRE-1 is unable to initialize this mechanism. Rather, the Nrf2 pathway is activated, which could initiate stress resistance.
Some case reports have also indicated that excessive intake of tea extracts induces liver toxicity [183], which is probably due to the prooxidant property of tea polyphenols [184]. It is proposed that low and moderate doses of tea polyphenols produce lower levels of ROS, which activates Nrf2 to attenuate oxidative stress, whilst high dose of tea polyphenols produce high levels of ROS and induce toxicity [185]. In this regard, optimum doses of green tea must be consumed, which can provide numerous health benefits to mankind.
NF-κB, a family of inducible transcription factors, regulates a large array of genes involved in immune and inflammatory responses [41]. In alveolar macrophages, NF-κB activation can induce the production of IL-6, TNF-α, IL-1β, MIP-2, and iNOS. In the present study, CSE and LPS treatment increased the production of IL-6, TNF-α, MIP-2, iNOS, and NO (Figure 5b–e). Furthermore, treatment with CSE and LPS activated the NF-κB pathway by degrading IκB in the cytosol and translocating p65 into the nucleus. In contrast, CLE induced the inactivation of NF-κB (Figure 5f), resulting in the suppression of the production of pro-inflammatory cytokines, chemokines, iNOS, and NO (Figure 5b–e). Another crucial pathway is NRF2/HO-1, an antioxidant defense system.
NRF2 is located in the cytoplasm where it is bound to the inhibitory protein Keap1 under normal physiological conditions [42]. Under oxidative stress, NRF2 is released from Keap1, and translocated into the nucleus. Next, NRF2 binds to antioxidant response elements (ARE) to regulate the transcription of antioxidant genes such as the inducible enzyme HO-1 [43]. HO-1 plays a pivotal role in host defense against oxidative stress [44]. Therefore, the NRF2/HO-1 pathway was investigated to confirm the anti-inflammatory and antioxidant effects of CLE. We analyzed the expression of NRF2 and HO-1 after CLE treatment in CSE- and LPS-stimulated MH-S. As shown in Figure 5f, the CLE induced NRF2 translocation in a dose-dependent manner. Accordingly, HO-1 production increased in a dose-dependent manner following CLE treatment. Based on the above results, we suggest that CLE treatment induced the activation of the NRF2/HO-1 signaling pathway and that the NF-κB/IL-6/MIP-2 signaling pathway was suppressed by CLE.
Guggulsterone
Key findings: Z-GS significantly increased the body weights and bone mineral density, ameliorated the femoral biomechanical parameters and microstructure of GIO rats. Z-GS treatment also reversed DXM-induced changes of bone turnover markers and oxidative stress in rats and MC3T3-E1 cells. The expressions of Nrf2 and HO-1 were inhibited in the model group and treatment with Z-GS could markedly increase their expressions. Nrf2 or HO-1 knockdown observably abrogated the beneficial role of Z-GS on cells.
Significance: Our results demonstrated that Z-GS exerted bone protective and antioxidant stress properties through activation of Nrf2/HO-1 signaling in GIO models in vivo and in vitro. Therefore, Z-GS could be considered as a promising candidate for the treatment of GIO.
Guggulsterone (GS) [4,17(20)-pregnadiene-3,16-dione] is a phytosterol found in the gum resin of the Commiphora mukul. GS exists naturally in two stereoisomers: E-GS (cis-GS) and Z-GS (trans-GS). In this study, the effects of both isomers on expression of the cytoprotective enzyme heme oxygenase-1 (HO-1) were evaluated in human mammary epithelial (MCF10A) cells. NF-E2-related factor 2 (Nrf2) is considered a master regulator in activating antioxidant response element (ARE)-driven expression of HO-1 and many other antioxidant/cytoprotective proteins. cis-GS upregulated the transcription and protein expression of HO-1 to a greater extent than did trans-GS. cis-GS treatment enhanced nuclear translocation and ARE-binding activity of Nrf2. MCF10A cells transfected with an ARE luciferase construct exhibited significantly elevated Nrf2 transcriptional activity upon cis-GS treatment compared with cells transfected with the control vector. In addition, silencing of the Nrf2 gene abrogated cis-GS-induced expression of HO-1. Incubation of MCF10A cells with cis-GS increased phosphorylation of Akt. The pharmacological inhibition of phosphoinositide 3-kinase (PI3K), an upstream kinase responsible for Akt phosphorylation, abrogated cis-GS-induced Nrf2 nuclear translocation. Pretreatment with the thiol-reducing agents attenuated Akt phosphorylation, Nrf2 activation and HO-1 expression, suggesting that cis-GS may cause thiol modification of an upstream signaling modulator. Phosphatase and Tensin Homologue Deleted on Chromosome 10 (PTEN) is a negative regulator of the PI3K-Akt axis. The mutation in cysteine 124 present in the catalytic domain of PTEN abolished cis-GS-induced HO-1 expression as well as Akt phosphorylation. Whether this cysteine is a ‘bona fide’ target of cis-GS in its activation of Nrf2 needs additional investigation.
(E)-Guggulsterone Inhibits Dengue Virus Replication by Upregulating Antiviral Interferon Responses
Dengue virus (DENV) infection, which causes dengue fever, dengue hemorrhagic fever, and dengue shock syndrome, is a severe global health problem in tropical and subtropical areas. There is no effective vaccine or drug against DENV infection. Thus, the development of anti-DENV agents is imperative. This study aimed to assess the anti-DENV activity of (E)-guggulsterone using a DENV infectious system. A specific inhibitor targeting signal molecules was used to evaluate the molecular mechanisms of action. Western blotting and qRT-PCR were used to determine DENV protein expression and RNA replication, respectively. Finally, an ICR suckling mouse model was used to examine the anti-DENV activity of (E)-guggulsterone in vivo. A dose-dependent inhibitory effect of (E)-guggulsterone on DENV protein synthesis and RNA replication without cytotoxicity was observed. The mechanistic studied revealed that (E)-guggulsterone stimulates Nrf2-mediated heme oxygenase-1 (HO-1) expression, which increases the antiviral interferon responses and downstream antiviral gene expression by blocking DENV NS2B/3B protease activity. Moreover, (E)-guggulsterone protected ICR suckling mice from life-threatening DENV infection. These results suggest that (E)-guggulsterone can be a potential supplement for controlling DENV replication.
Hinokitiol
Hinokitiol functions as a ferroptosis inhibitor to confer neuroprotection
The intrinsic link of ferroptosis to neurodegeneration, such as Parkinson’s disease and Alzheimer’s disease, has set promises to apply ferroptosis inhibitors for treatment of neurodegenerative disorders. Herein, we report that the natural small molecule hinokitiol (Hino) functions as a potent ferroptosis inhibitor to rescue neuronal damages in vitro and in vivo. The action mechanisms of Hino involve chelating irons and activating cytoprotective transcription factor Nrf2 to upregulate the antioxidant genes including solute carrier family 7 member 11, glutathione peroxidase 4 and Heme oxygenase-1. In vivo studies demonstrate that Hino rescues the deficits of locomotor activity and neurodevelopment in zebrafishes. In addition, Hino shows the efficient blood-brain barrier permeability in mice, supporting the application of Hino for brain disorders. Paclitaxel is one of the most widely used broad-spectrum antineoplastic agents. However, its neurotoxic side effect is a severe concern. We demonstrate that the neurotoxicity of paclitaxel is ferroptosis-related and Hino also alleviates the paclitaxel-induced neurotoxicity without compromising its cytotoxicity to cancer cells. Hino also salvages the neurobehavioral impairment by paclitaxel in zebrafishes.
Hinokitiol suppresses cancer stemness and oncogenicity in glioma stem cells by Nrf2 regulation
Results: We demonstrated that hinokitiol effectively inhibited the CD133 positivity and ALDH1 activity along with the reduced self-renewal, migration, invasion, and colony formation properties of GSCs. In addition, hinokitiol repressed the gene and protein expression of Nrf2, which has been shown to be critical for those GSCs features. Furthermore, we showed that administration of exogenous Nrf2 counteracted the inhibitory effect of hinokitiol on self-renewal and invasiveness of GSCs.
Conclusion: These evidences suggest that treatment of hinokitiol significantly attenuates the hallmarks of GSCs due to downregulation of Nrf2 expression. Hence, hinokitiol may serve as a promising agent for the therapy of glioma.
Hydroxytyrosol
Hydroxytyrosol (HTy) is a natural polyphenol abundant in olive oil, which possesses multiple biological actions. Particularly, HTy has cytoprotective activity against oxidative-stress-induced cell damage, but the underlying mechanisms of action remain unclear. Here, we have investigated the molecular mechanism involved in the protection exerted by HTy on tert-butyl hydroperoxide-induced damage in human HepG2 liver cells. Treatment of HepG2 cells with HTy increased the expression and the activity of glutathione-related enzymes such as glutathione peroxidase, glutathione reductase and glutathione S-transferase. HTy also induced the nuclear transcription factor erythroid 2p45-related factor (Nrf2), a transcription factor implicated in the expression of several antioxidant/detoxificant enzymes. Moreover, two important signalling proteins involved in Nrf2 translocation, the protein kinase B and the extracellular regulated kinases, were also activated by HTy. Further studies with specific inhibitors confirmed that both molecular pathways are critical for the nuclear translocation of Nrf2, the increased enzyme expression and activity and the beneficial effect against oxidative stress induced by HTy. In conclusion, together with the inherent radical scavenging activity of HTy, our results provide an additional mechanism of action to prevent oxidative stress damage through the modulation of signalling pathways involved in antioxidant/detoxifying enzymes regulation.
This study was conducted to investigate the antioxidant effects of hydroxytyrosol (HT) administration in diquat (DQ)-challenged mice. The results showed that HT treatment markedly alleviated DQ-induced oxidative stress, which was indicated by the enhanced total antioxidant capacity (T-AOC), increased activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase and decreased malondialdehyde (MDA) concentration in serum. Additionally, HT increased the mRNA expression levels of NF-E2-related factor 2 (Nrf2) and its downstream genes, including NADPH quinone oxidoreductase 1 (NQO1) and catalase (CAT) in the small intestine of DQ-challenged mice. 16S rRNA gene sequencing results showed that HT treatment increased the relative abundance of Firmicutes and Lactobacillus and decreased the relative abundance of Bacteroidetes. Interestingly, Pearson correlation analysis showed that there were strong association between colonic Firmicutes, Lactobacillus, and Bacteroidetes and the activities of serum antioxidant enzymes. Meanwhile, HT significantly enhanced the colonic butyrate concentration in DQ-challenged mice. Additionally, HT treatment decreased the serum metabolites involving in glycerophospholipid metabolism, pentose, and glucuronate interconversions, which were associated with alleviated oxidative stress. These results indicate that oral administration of 100 mg/kg body weight HT alleviates oxidative stress in DQ-challenged mice, which may involve Nrf2 signaling pathways via modulation of colonic microbiota.
Hyperoside
In our ongoing research to discover natural products with neuroprotective effects, hyperoside (quercetin 3-O-galactoside) was isolated from Acer tegmentosum, which has been used in Korean traditional medicine to treat liver-related disorders. Here, we demonstrated that hyperoside protects cultured dopaminergic neurons from death via reactive oxygen species (ROS)-dependent mechanisms, although other relevant mechanisms of hyperoside activity remain largely uncharacterized. For the first time, we investigated the neuroprotective effects of hyperoside on 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in neurons, and the possible underlying mechanisms. Hyperoside significantly ameliorated the loss of neuronal cell viability, lactate dehydrogenase release, excessive ROS accumulation and mitochondrial membrane potential dysfunction associated with 6-OHDA-induced neurotoxicity. Furthermore, hyperoside treatment activated the nuclear erythroid 2-related factor 2 (Nrf2), an upstream molecule of heme oxygenase-1 (HO-1). Hyperoside also induced the expression of HO-1, an antioxidant response gene. Remarkably, we found that the neuroprotective effects of hyperoside were weakened by an Nrf2 small interfering RNA, which blocked the ability of hyperoside to inhibit neuronal death, indicating the vital role of HO-1. Overall, we show that hyperoside, via the induction of Nrf2-dependent HO-1 activation, suppresses neuronal death caused by 6-OHDA-induced oxidative stress. Moreover, Nrf2-dependent HO-1 signaling activation represents a potential preventive and therapeutic target in Parkinson′s disease management.
Hyperoside Induces Endogenous Antioxidant System to Alleviate oxidative stress
Results: Hyperoside increased both the mRNA and protein expression of HO-1 in a time- and dose-dependent manner. In addition, hyperoside elevated the level of of Nrf2 and its antioxidant response element-binding activity, which was modulated by upstream of ERK. Moreover, it activated ERK and restored cell viability which was decreased by hydrogen peroxide.
Conclusions: Hyperoside is an effective compound to protect cells against oxidative stress via HO-1 induction.
Glycogen synthase kinase-3β (GSK-3β) acts as a negative regulator of NF-E2 related factor 2 (Nrf2) by inducing Nrf2 degradation and nuclear export. Our previous study demonstrated that the flavonoid hyperoside elicits cytoprotection against oxidative stress by activating the Keap1-Nrf2-ARE signaling pathway, thus increasing the expression of antioxidant enzymes, such as heme oxygenase-1 (HO-1), superoxide dismutase (SOD) and catalase. However, the role of GSK-3β in hyperoside-mediated Nrf2 activation is unclear. Here, we demonstrate that in a normal human hepatocyte cell line, (L02), hyperoside is capable of inducing the phosphorylation of GSK-3β at Ser9 without affecting the protein levels of GSK-3β and its phosphorylation at Thr390. Lithium chloride (LiCl) and short interfering RNA (siRNA)-mediated inhibition of GSK-3β significantly enhanced the ability of hyperoside to protect L02 liver cells from H2O2-induced oxidative damage, leading to increased cell survival shown by the maintenance of cell membrane integrity and elevated levels of glutathione (GSH), one of the endogenous antioxidant biomarkers.
Further study showed that LiCl and siRNA-mediated inhibition of GSK-3β increased hyperoside-induced HO-1 expression, and the effect was dependent upon enhanced Nrf2 nuclear translocation and gene expression. These activities were followed by ARE-mediated transcriptional activation in the presence of hyperoside, which was abolished by the transfection of the cells with Nrf2 siRNA. Furthermore, the siRNA-mediated inhibition of Keap1 also enhanced hyperoside-induced Nrf2 nuclear accumulation and HO-1 expression, which was relatively smaller than the effects obtained from GSK-3β siRNA administration. Moreover, Keap1 siRNA administration alone had no significant effect on the phosphorylation and protein expression of GSK-3β. Collectively, our data provide evidence that hyperoside attenuates H2O2 -induced L02 cell damage by activating the Nrf2-ARE signaling pathway through both an increase in GSK-3β inhibitory phosphorylation at Ser9 and an inhibition of Keap1 and that hyperoside-mediated GSK-3β inhibition exhibits more significant effects.
Isoorientin
Isoorientin Ameliorates APAP-Induced Hepatotoxicity via Activation Nrf2 Antioxidative Pathway
The present study investigated the hepatoprotective effect of Iso and its underlying mechanism. C57BL/6J mice were used to evaluate the hepatoprotective effect of Iso in vivo and HepG2 cells were utilized to further decipher the mechanisms of Iso-induced Nrf2 activation. We found that Iso treatment significantly reduced APAP-induced hepatotoxicity by reducing the lethality, histopathological liver changes, and alanine transaminase (ALT) and aspartate aminotransferase (AST) levels in serum. These effects were accompanied by decreased malondialdehyde (MDA) formation and myeloperoxidase level (MPO), and by decreased superoxide dismutase (SOD) and glutathione (GSH) depletion.
Moreover, Iso induced Nrf2 activation and translocation as well as upstream AMPK/Akt/GSK3β activation. Furthermore, Iso effectively alleviated mitochondrial dysfunction by reducing c-jun N-terminal kinase phosphorylation and translocation, Bax mitochondrial translocation, and apoptosis-inducing factor and cytochrome c release. Further mechanistic investigations revealed that the activation of Nrf2 by Iso via the AMPK/Akt/GSK3β pathway contributed to the hepatoprotective activity of Iso in vitro. In addition, the Iso-mediated inhibition of APAP-induced the lethality, histopathological changes and mitochondrial dysfunction observed in WT mice was nearly absent in Nrf2-/- mice. In summary, Iso ameliorated APAP-induced hepatotoxicity by activating Nrf2 via the AMPK/Akt/GSK3β pathway.
Kaempferol
Kaempferol is a natural flavonoid, which has been widely investigated in the treatment of cancer, cardiovascular diseases, metabolic complications, and neurological disorders. Nrf2 (nuclear factor erythroid 2-related factor 2) is a transcription factor involved in mediating carcinogenesis and other ailments, playing an important role in regulating oxidative stress. The activation of Nrf2 results in the expression of proteins and cytoprotective enzymes, which provide cellular protection against reactive oxygen species. Phytochemicals, either alone or in combination, have been used to modulate Nrf2 in cancer and other ailments. Among them, kaempferol has been recently explored for its anti-cancer and other anti-disease therapeutic efficacy, targeting Nrf2 modulation.
In combating cancer, diabetic complications, metabolic disorders, and neurological disorders, kaempferol has been shown to regulate Nrf2 and reduce redox homeostasis. In this context, this review article highlights the current status of the therapeutic potential of kaempferol by targeting Nrf2 modulation in cancer, diabetic complications, neurological disorders, and cardiovascular disorders. In addition, we provide future perspectives on kaempferol targeting Nrf2 modulation as a potential therapeutic approach.
Kinsenoside
Intervertebral disc degeneration (IDD) is recognized as the major contributor to low back pain, which results in disability worldwide and heavy burdens on society and economy. Here we present evidence that the lower level of Nrf2 is closely associated with higher grade of IDD. The apoptosis and senescence of nucleus pulposus cells (NPCs) were exacerbated by Nrf2 knockdown, but suppressed by Nrf2 overexpression under oxidative stress. Based on findings that Kinsenoside could exert multiple pharmacological effects, we found that Kinsenoside rescued the NPC viability under oxidative stress and protected against apoptosis, senescence and mitochondrial dysfunction in a Nrf2-dependent way. Further experiments revealed that Kinsenoside activated a signaling pathway of AKT-ERK1/2-Nrf2 in NPCs. Moreover, in vivo study showed that Kinsenoside ameliorated IDD in a puncture-induced model. Together, the present work suggests that Nrf2 is involved in the pathogenesis of IDD and shows the protective effects as well as the underlying mechanism of Kinsenoside on Nrf2 activation in NPCs.
The Nrf2 antioxidant defense system in intervertebral disc degeneration: Molecular insights
Intervertebral disc degeneration (IDD) is a common degenerative musculoskeletal disorder and is recognized as a major contributor to discogenic lower back pain. However, the molecular mechanisms underlying IDD remain unclear, and therapeutic strategies for IDD are currently limited. oxidative stress plays pivotal roles in the pathogenesis and progression of many age-related diseases in humans, including IDD. Nuclear factor E2-related factor 2 (Nrf2) is a master antioxidant transcription factor that protects cells against oxidative stress damage. Nrf2 is negatively modulated by Kelch-like ECH-associated protein 1 (Keap1) and exerts important effects on IDD progression. Accumulating evidence has revealed that Nrf2 can facilitate the transcription of downstream antioxidant genes in disc cells by binding to antioxidant response elements (AREs) in promoter regions, including heme oxygenase-1 (HO-1), glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and NADPH quinone dehydrogenase 1 (NQO1). The Nrf2 antioxidant defense system regulates cell apoptosis, senescence, extracellular matrix (ECM) metabolism, the inflammatory response of the nucleus pulposus (NP), and calcification of the cartilaginous endplates (EP) in IDD. In this review, we aim to discuss the current knowledge on the roles of Nrf2 in IDD systematically.
The disorder of mitochondrial dynamic equilibrium of lung epithelial cell is one of the critical causes of acute lung injury (ALI). Kinsenoside (Kin) serves as an active small-molecule component derived from traditional medicinal herb displaying multiple pharmacological actions in cancers, hyperglycemia, and liver disease. The objective of this study was to investigate the effects of Kin on lipopolysaccharide- (LPS-) induced ALI and further explore possible molecular mechanisms. Kin was administered orally (100 mg/kg/day) for 7 consecutive days before LPS instillation (5 mg/kg). After 12 hours, pathological injury, inflammatory response, and oxidative stress were detected. The results demonstrated that Kin significantly alleviated lung pathological injury and decreased the infiltration of inflammatory cells and the release of inflammatory mediators in bronchoalveolar lavage fluid (BALF), apart from inhibiting the production of reactive oxygen species (ROS) and lipid peroxidation. Meanwhile, Kin also promoted mitochondrial fusion and restrained mitochondrial fission in mice with ALI. In terms of mechanism, Kin pretreatment increased the phosphorylation of AMP-activated protein kinase (AMPK) and the protein level of nuclear factor erythroid 2-related factor 2 (Nrf2). In Ampk-α knockout mice challenged with LPS, Kin lost its pulmonary protective effects, accompanied by lower Nrf2 level. In vitro experiments further unveiled that either AMPK inhibition by Compound C or Nrf2 knockdown by siRNA abolished the protective roles of Kin in LPS-treated A549 lung epithelial cells. And Nrf2 activator TAT-14 could reverse the effects of Ampk-α deficiency. In conclusion, Kin possesses the ability to prevent LPS-induced ALI by modulating mitochondrial dynamic equilibrium in lung epithelial cell in an AMPK/Nrf2-dependent manner.
L-Epicatechin
(-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways
EC significantly reduced lesion volume and ameliorated neurologic deficits in both male and female ICH mice. Cell death and neuronal degeneration were decreased in the perihematomal area and were associated with reductions in caspase-3 activity and high-mobility group protein B1 (HMGB-1) level. These changes were accompanied by attenuation of oxidative insults, increased phase II enzyme expression, and increased Nrf2 nuclear accumulation. Interestingly, in addition to providing neuroprotection via Nrf2 signaling, EC diminished heme oxygenase-1 induction and brain iron deposition via an Nrf2-independent pathway that downregulated ICH-induced activating protein-1 activation and decreased matrix metalloproteinase 9 activity, lipocalin-2 levels, iron-dependent cell death, and ferroptosis-related gene expression.
Collectively, our data show that EC protects against ICH by activation of Nrf2-dependent and -independent pathways and may serve as a potential intervention for patients with ICH.
Traumatic brain injury (TBI), which leads to disability, dysfunction, and even death, is a prominent health problem worldwide with no effective treatment. A brain-permeable flavonoid named (−)-epicatechin (EC) modulates redox/ oxidative stress and has been shown to be beneficial for vascular and cognitive function in humans and for ischemic and hemorrhagic stroke in rodents. Here we examined whether EC is able to protect the brain against TBI-induced brain injury in mice and if so, whether it exerts neuroprotection by modulating the NF-E2-related factor (Nrf2) pathway. We used the controlled cortical impact model to mimic TBI. EC was administered orally at 3 h after TBI and then every 24 h for either 3 or 7 days. We evaluated lesion volume, brain edema, white matter injury, neurologic deficits, cognitive performance and emotion-like behaviors, neutrophil infiltration, reactive oxygen species (ROS), and a variety of injury-related protein markers. Nrf2 knockout mice were used to determine the role of the Nrf2 signaling pathway after EC treatment. In wild-type mice, EC significantly reduced lesion volume, edema, and cell death and improved neurologic function on days 3 and 28; cognitive performance and depression-like behaviors were also improved with EC administration. In addition, EC reduced white matter injury, heme oxygenase-1 expression, and ferric iron deposition after TBI. These changes were accompanied by attenuation of neutrophil infiltration and oxidative insults, reduced activity of matrix metalloproteinase 9, decreased Keap 1 expression, increased Nrf2 nuclear accumulation, and increased expression of superoxide dismutase 1 and quinone 1. However, EC did not significantly reduce lesion volume or improve neurologic deficits in Nrf2 knockout mice after TBI. Our results show that EC protects the TBI brain by activating the Nrf2 pathway, inhibiting heme oxygenase-1 protein expression, and reducing iron deposition. The latter two effects could represent an Nrf2-independent mechanism in this model of TBI.
The Flavanol (−)-Epicatechin Prevents Stroke Damage through the Nrf2/HO1 Pathway
In this study, we investigated whether epicatechin (EC), a flavanol in cocoa and tea, is protective against brain ischemic damage in mice. Wild-type mice pretreated orally with 5, 15, or 30 mg/kg EC before middle cerebral artery occlusion (MCAO) had significantly smaller brain infarcts and decreased neurologic deficit scores (NDS) than did the vehicle-treated group. Mice that were posttreated with 30 mg/kg of EC at 3.5 hours after MCAO also had significantly smaller brain infarcts and decreased NDS. Similarly, WT mice pretreated with 30 mg/kg of EC and subjected to N-methyl-D-aspartate (NMDA)-induced excitotoxicity had significantly smaller lesion volumes. Cell viability assays with neuronal cultures further confirmed that EC could protect neurons against oxidative insults. Interestingly, the EC-associated neuroprotection was mostly abolished in mice lacking the enzyme heme oxygenase 1 (HO1) or the transcriptional factor Nrf2, and in neurons derived from these knockout mice. These results suggest that EC exerts part of its beneficial effect through activation of Nrf2 and an increase in the neuroprotective HO1 enzyme.
Lutein
Lutein as a Modulator of oxidative stress-Mediated Inflammatory Diseases
Lutein is a xanthophyll carotenoid obtained from various foods, such as dark green leafy vegetables and egg yolk. Lutein has antioxidant activity and scavenges reactive oxygen species such as singlet oxygen and lipid peroxy radicals. oxidative stress activates inflammatory mediators, leading to the development of metabolic and inflammatory diseases. Thus, recent basic and clinical studies have investigated the anti-inflammatory effects of lutein based on its antioxidant activity and modulation of oxidant-sensitive inflammatory signaling pathways. Lutein suppresses activation of nuclear factor-kB and signal transducer and activator of transcription 3, and induction of inflammatory cytokines (interleukin-1β, interleukin-6, monocyte chemoattratant protein-1, tumor necrosis factor-α) and inflammatory enzymes (cyclooxygenase-2, inducible nitric oxide synthase). It also maintains the content of endogenous antioxidant (glutathione) and activates nuclear factor erythroid 2–related factor 2 (Nrf2) and Nrf2 signaling-related antioxidant enzymes (hemeoxygenase-1, NAD(P)H: quinone oxidoreductase 1, glutathione-s-transferase, glutathione peroxidase, superoxide dismutase, catalase). In this review, we have discussed the current knowledge regarding the anti-inflammatory function of lutein against inflammatory diseases in various organs, including neurodegenerative disorders, eye diseases, diabetic retinopathy, osteoporosis, cardiovascular diseases, skin diseases, liver injury, obesity, and colon diseases.
Lutein activates the Transcription Factor Nrf2 in Human Retinal Pigment Epithelial Cells
The degeneration of the retinal pigment epithelium caused by oxidative damage is a stage of development in age-related macular degeneration (AMD). The carotenoid lutein is a major macular pigment that may reduce the incidence and progression of AMD, but the underlying mechanism is currently not fully understood. Carotenoids are known to be direct antioxidants. However, carotenoids can also activate cellular pathways resulting in indirect antioxidant effects. Here, we investigate the influence of lutein on the activation of nuclear factor erythroid 2-related factor 2 (Nrf2) target genes in human retinal pigment epithelial cells (ARPE-19 cells) using lutein-loaded Tween40 micelles. The micelles were identified as a suitable delivery system since they were nontoxic in APRE-19 cells up to 0.04% Tween40 and led to a cellular lutein accumulation of 62 μM ± 14 μM after 24 h. Lutein significantly enhanced Nrf2 translocation to the nucleus 1.5 ± 0.4-fold compared to that of unloaded micelles after 4 h. Furthermore, lutein treatment for 24 h significantly increased the transcripts of NAD(P)H:quinone oxidoreductase 1 (NQO1) by 1.7 ± 0.1-fold, glutamate-cysteine ligase regulatory subunit (GCLm) by 1.4 ± 0.1-fold, and heme oxygenase-1 (HO-1) by 1.8 ± 0.3-fold. Moreover, we observed a significant enhancement of NQO1 activity by 1.2 ± 0.1-fold. Collectively, this study indicates that lutein not only serves as a direct antioxidant but also activates Nrf2 in ARPE-19 cells.
Luteolin
Colorectal cancer remains a leading malignancy in humans. The importance of epigenetic modification in the development of this disease is now being recognized. The reversible and dynamic nature of epigenetic modifications provides a promising strategy in colorectal cancer chemoprevention and treatment. Luteolin (LUT), a flavone dietary phytochemical, can modulate various signaling pathways involved in carcinogenesis. Many studies have demonstrated that LUT inhibits colorectal carcinogenesis by activating the Nrf2/ARE pathway. However, the potential epigenetic mechanism underlying Nrf2/ARE pathway activation remains unclear. In this study, we aimed to explore the anticancer potential of LUT in human colon cancer cells and the epigenetic regulation of the Nrf2/ARE pathway.
Specifically, our data showed that LUT suppressed cell proliferation and cellular transformation of HCT116 and HT29 cells in a dose-dependent manner. Additionally, qPCR and western blotting were performed to determine the mRNA and protein expression of Nrf2 and its downstream genes after LUT treatment. Bisulfite genomic sequencing revealed that methylation of the Nrf2 promoter region was decreased by LUT, corresponding with the increased mRNA expression of Nrf2. Decreased protein levels and enzyme activities of epigenetic modifying enzymes, such as DNMTs and HDACs, were also observed in LUT-treated HCT116 cells. In summary, our findings suggest that LUT may exert its anti-tumor activity in part via epigenetic modifications of the Nrf2 gene with subsequent induction of its downstream antioxidative stress pathway.
lycopene
In alcoholic pancreatitis, alcohol increases gut permeability, which increases the penetration of endotoxins, such as lipopolysaccharides (LPS). LPS act as clinically significant triggers to increase pancreatic damage in alcoholic pancreatitis. Ethanol or LPS treatment increases reactive oxygen species (ROS) production in pancreatic acinar cells. ROS induce inflammatory cytokine production in pancreatic acinar cells, leading to pancreatic inflammation. The nuclear erythroid-2-related factor 2 (Nrf2) pathway is activated as a cytoprotective response to oxidative stress, and induces the expression of NAD(P)H quinone oxidoreductase 1 (NQO1) and heme oxygenase-1 (HO-1). Lycopene exerts anti-inflammatory and antioxidant effects in various cells. We previously showed that lycopene inhibits NADPH oxidase to reduce ROS and IL-6 levels, and zymogene activation in ethanol or palmitoleic acid-treated pancreatic acinar cells. In this study, we examined whether lycopene inhibits IL-6 expression by activating the Nrf2/NQO1-HO-1 pathway, and reducing intracellular and mitochondrial ROS levels, in ethanol and LPS-treated pancreatic AR42J cells. Lycopene increased the phosphorylated and nuclear-translocated Nrf2 levels by decreasing the amount of Nrf2 sequestered in the cytoplasm via a complex formation with Kelch-like ECH1-associated protein 1 (Keap1). Using exogenous inhibitors targeting Nrf2 and HO-1, we showed that the upregulation of activated Nrf2 and HO-1 results in lycopene-induced suppression of IL-6 expression and ROS production. The consumption of lycopene-rich foods may prevent the development of ethanol and LPS-associated pancreatic inflammation by activating Nrf2-mediated expression of NQO1 and HO-1, thereby decreasing ROS-mediated IL-6 expression in pancreatic acinar cells.
Lycopene can be cleaved by carotene 9′,10′-oxygenase at its 9′,10′ double bond to form apo-10′-lycopenoids, including apo-10′-lycopenal, -lycopenol and -lycopenoic acid. The latter has been recently shown to inhibit lung carcinogenesis both in vivo and in vitro, however, the mechanism(s) underlying this protection is not well defined. In the present study, we report that treatment with apo-10′-lycopenoic acid, in a time- and dose-dependent manner, results in the nuclear accumulation of transcription factor Nrf2 (nuclear factor E2-related factor 2) protein in BEAS-2B human bronchial epithelial cells. The activation of Nrf2 by apo-10′-lycopenoic acid is associated with the induction of phase II detoxifying/antioxidant enzymes including heme oxygenase-1, NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate–cysteine ligases in BEAS-2B cells. Furthermore, apo-10′-lycopenoic acid treatment increased total intracellular glutathione levels and suppressed both endogenous reactive oxygen species generation and H2O2-induced oxidative damage in BEAS-2B cells. In addition, both apo-10′-lycopenol and apo-10′-lycopenal induced heme oxygenase-1 gene expression in BEAS-2B cells. These data strongly suggest that the anti-carcinogenic and antioxidant functions of lycopene may be mediated by apo-10′-lycopenoids via activating Nrf2 and inducing phase II detoxifying/antioxidant enzymes.
In alcoholic pancreatitis, alcohol increases gut permeability, which increases the penetration of endotoxins, such as lipopolysaccharides (LPS). LPS act as clinically significant triggers to increase pancreatic damage in alcoholic pancreatitis. Ethanol or LPS treatment increases reactive oxygen species (ROS) production in pancreatic acinar cells. ROS induce inflammatory cytokine production in pancreatic acinar cells, leading to pancreatic inflammation. The nuclear erythroid-2-related factor 2 (Nrf2) pathway is activated as a cytoprotective response to oxidative stress, and induces the expression of NAD(P)H quinone oxidoreductase 1 (NQO1) and heme oxygenase-1 (HO-1). Lycopene exerts anti-inflammatory and antioxidant effects in various cells. We previously showed that lycopene inhibits NADPH oxidase to reduce ROS and IL-6 levels, and zymogene activation in ethanol or palmitoleic acid-treated pancreatic acinar cells. In this study, we examined whether lycopene inhibits IL-6 expression by activating the Nrf2/NQO1-HO-1 pathway, and reducing intracellular and mitochondrial ROS levels, in ethanol and LPS-treated pancreatic AR42J cells. Lycopene increased the phosphorylated and nuclear-translocated Nrf2 levels by decreasing the amount of Nrf2 sequestered in the cytoplasm via a complex formation with Kelch-like ECH1-associated protein 1 (Keap1). Using exogenous inhibitors targeting Nrf2 and HO-1, we showed that the upregulation of activated Nrf2 and HO-1 results in lycopene-induced suppression of IL-6 expression and ROS production. The consumption of lycopene-rich foods may prevent the development of ethanol and LPS-associated pancreatic inflammation by activating Nrf2-mediated expression of NQO1 and HO-1, thereby decreasing ROS-mediated IL-6 expression in pancreatic acinar cells.
Myricetin
This study aimed to investigate the protective effects and mechanisms of myricetin on acute liver failure in mice induced by lipopolysaccharide (LPS)/D-galactosamine (D-Gal). Our results showed myricetin (25, 50 and 100 mg/kg) pretreatment significantly improved the pathological changes of liver tissues, decreased serum ALT and AST (p < 0.001) induced by LPS/D-GalN. Moreover, MDA and MPO levels were reduced (p < 0.001), CAT and SOD activities were increased (p < 0.001) with myricetin (50 and 100 mg/kg) pretreatment. Likewise, inflammatory cytokines TNF-α and IL-6 mRNA in liver tissues were markedly decreased (p < 0.001) by myricetin. Besides, Nrf2 protein expression was drastically elevated (p < 0.001) by myricetin (25, 50 and 100 mg/kg). All these findings imply that myricetin may protect against acute liver failure by suppressing inflammation and regulating oxidative stress via Nrf2 signaling, and that it may be a possible strategy to avoid liver damage.
Results: Myricetin not only inhibited the generation of inflammatory mediators and cytokines such as nitric oxide (NO), prostaglandin E2 (PGE2), TNF-α and IL-6, but also suppressed the production of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in human chondrocytes under IL-1β stimulation. Moreover, Metalloproteinase 13 (MMP13) and thrombospondin motifs 5 (ADAMTS5), which resulted in the degradation of cartilage, were also suppressed in chondrocytes with the treatment of myricetin. To explore the potential mechanism, we found out that myricetin suppressed NF-κB signaling pathway through Nrf2/HO-1 axis in human chondrocytes. Besides, myricetin regulated the Nrf2 signaling pathway through PI3K/Akt pathway. In addition, in vivo study demonstrated that myricetin could ameliorated the progression of OA in mice DMM model through PI3K/Akt mediated Nrf2 signaling pathway.
Conclusion: Taken together, our data first demonstrated that myricetin possesses the therapeutic potential on OA through PI3K/Akt mediated Nrf2/HO-1 signaling pathway.
The aim of our study was to investigate the protective effects and underlying mechanisms of myricetin, a bioactive food compound, on brain injury and neurological deficits after ischemic stroke. Treatment of myricetin significantly attenuated oxygen-glucose deprivation (OGD)-induced cell death in SHSY5Y cells in vitro. In a rat model of cerebral ischemia, myricetin was administered intragastrically at 2 h before and every day after middle cerebral artery occlusion (MCAO). The effects of myricetin were evaluated by various biochemical assays and neurobehavioral tests. Treatment with myricetin resulted in decreased infarction volume, reduced neuronal loss as well as lessened production of reactive oxygen species (ROS) and malondialdehyde following MCAO. We also found evidence that myricetin treatment could enhance the activity of antioxidant enzymes and mitochondrial function. Meanwhile, myricetin treatment reversed the suppression of Nrf2 nuclear translocation, and increased HO-1 expression in the ipsilateral ischemic brain and in the normal brain. Moreover, our results suggested that myricetin treatment resulted in significant improvement in neurological function. In conclusion, treatment with myricetin attenuates brain injury and neurological deficits in a rat model of cerebral ischemia via improvement of mitochondrial function and activation of the Nrf2 pathway.
Naringenin
Oxidative stress and mitochondrial dysfunction are considered to be major contributing factors in the development and progression of many neurodegenerative diseases. Naringenin (NAR) is an abundant flavanone in the Citrus genus and has been found to exert antioxidant, anticarcinogenic and antimutagenic effects. However, the potential underlying mechanism of its antioxidant effects remains unclear. In the present study, the authors investigated the antioxidant effect of NAR on neurons in vitro. Neurons isolated from the brains of Sprague-Dawley rats were randomly divided into a control group, model group, NAR-L group, NAR-M group and NAR-H group. The model group received hypoxia and re-oxygenation treatment, and the NAR-L, NAR-M and NAR-H groups received 20, 40 and 80 µM NAR, respectively.
The levels of reactive oxygen species (ROS) in each group were detected by chloromethyl-2′,7’dichlorodihydro fluorescein diacetate staining, and differences in mitochondrial dysfunction were analyzed through measurement of mitochondrial membrane potential (∆ψm), adenine nucleotide translocase transport activity and adenine nucleotide levels. MTT and flow cytometry assays were also used to analyze cell proliferation and apoptosis, and the effects of NAR on the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) signaling pathway were investigated using small interfering RNA methods.
The authors detected an increased accumulation of ROS in the model group, and high-dose NAR could significantly reduce the levels of ROS. Furthermore, NAR could improve mitochondrial dysfunction, as indicated by increased levels of high-energy phosphates, enhanced mitochondrial ANT transport activity and increased mitochondrial membrane potential. Moreover, NAR increased cell viability and decreased the rate of cell apoptosis. NAR also increased the expression of Nrf2 and its downstream target genes. These findings demonstrated that NAR could reduce oxidative stress and improve mitochondrial dysfunction via activation of the Nrf2/ARE signaling pathway in neurons.
There is increasing evidence that oxidative stress is critically involved in the pathogenesis of Parkinson’s disease (PD), suggesting that pharmacological targeting of the antioxidant machinery may have therapeutic value. Naringenin, a natural flavonoid compound, has been reported to possess neuroprotective effect against PD related pathology; however the mechanisms underlying its beneficial effects are poorly defined. Thus, the purpose of the present study was to investigate the potential neuroprotective role of naringenin and to delineate its mechanism of action against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in models of PD both in vitro and in vivo.
Naringenin treatment resulted in an increase in nuclear factor E2-related factor 2 (Nrf2) protein levels and subsequent activation of antioxidant response element (ARE) pathway genes in SH-SY5Y cells and in mice. Exposure of SH-SY5Y cells to naringenin provided protection against 6-OHDA-induced oxidative insults that was dependent on Nrf2, since treatment with Nrf2 siRNA failed to block against 6-OHDA neurotoxicity or induce Nrf2-dependent cytoprotective genes in SH-SY5Y cells. In mice, oral administration of naringenin resulted in significant protection against 6-OHDA-induced nigrostriatal dopaminergic neurodegeneration and oxidative damage. Our results indicate that activation of Nrf2/ARE signaling by naringenin is strongly associated with its neuroprotective effects against 6-OHDA neurotoxicity and suggest that targeting the Nrf2/ARE pathway may be a promising approach for therapeutic intervention in PD.
Naringin
Results: Treatment of fructose-fed rats with naringin significantly decreased the hepatic TG and cholesterol content as well as serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities. Naringin treatment also decreased the hepatic expression of carbohydrate response element binding protein (ChREBP), sterol regulatory element-binding protein-1c (SREBP-1c) and nuclear SREBP-1c (nSREBP-1c) as well as enzymes involved in DNL (acetyl CoA carboxylase [ACC] and fatty acid synthase [FAS]) and an enzyme involved in TG synthesis (glycerol-3-phosphate acyltransferase 1 [GPAT-1] and diacylglycerol acyltransferase2 [DGAT2]) in fructose-fed rats. In addition, naringin induced a significant decrease in the hepatic expression of nuclear factor kappa B (NF-κB) and tumor necrosis factor α (TNF-α). Furthermore, naringin administration restored the expression of the antioxidant mediators nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and heme oxygenase-1 (HO-1) in the liver of fructose-fed rats.
Conclusion: These results demonstrate that oral administration of naringin protects against fructose-induced hepatic steatosis by decreasing DNL and TG synthesis. In addition, naringin could prevent NAFLD progression via targeting the Nrf2/HO-1 and the NF-κB/TNF-α pathways.
Neoxanthin
Natural Products as Modulators of Nrf2 signaling Pathway in Neuroprotection
Neurodegenerative diseases (NDs) affect the West due to the increase in life expectancy. Nervous cells accumulate oxidative damage, which is one of the factors that triggers and accelerates neurodegeneration. However, cells have mechanisms that scavenge reactive oxygen species (ROS) and alleviate oxidative stress (OS). Many of these endogenous antioxidant systems are regulated at the gene expression level by the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2). In the presence of prooxidant conditions, Nrf2 translocates to the nucleus and induces the transcription of genes containing ARE (antioxidant response element). In recent years, there has been an increase in the study of the Nrf2 pathway and the natural products that positively regulate it to reduce oxidative damage to the nervous system, both in in vitro models with neurons and microglia subjected to stress factors and in vivo models using mainly murine models. Quercetin, curcumin, anthocyanins, tea polyphenols, and other less studied phenolic compounds such as kaempferol, hesperetin, and icariin can also modulate Nrf2 by regulating several Nrf2 upstream activators. Another group of phytochemical compounds that upregulate this pathway are terpenoids, including monoterpenes (aucubin, catapol), diterpenes (ginkgolides), triterpenes (ginsenosides), and carotenoids (astaxanthin, lycopene). This review aims to update the knowledge on the influence of secondary metabolites of health interest on the activation of the Nrf2 pathway and their potential as treatments for NDs.
Methods and results: The cytotoxic effect of H2O2 and cytoprotective potential of β-carotene, lutein, and neoxanthin was analyzed by WST-1 assay. The intracellular ROS level and mitochondrial membrane potential (MMP) were measured using DCFH-DA (2′, 7′-dichlorofluorescin diacetate) and JC-10 MMP assay. The expression of anti-oxidant and apoptotic markers was measured by western blot analysis. Neoxanthin pretreatment exhibited better protection than β-carotene and lutein against cell death caused by H2O2. It significantly arrested H2O2-mediated elevation of intracellular ROS levels and protected MMP. The intracellular antioxidant enzymes HO-1 and SOD-2 were upregulated by neoxanthin pretreatment. Neoxanthin also activated the protein expression of redox-sensitive trans activation factors, Nrf2 and NF-kB. The cytoprotective effect of neoxanthin was associated with increased expression of the anti-apoptotic protein, Bcl-2 and decreased pro-apoptotic protein Bax.
Notoginsenoside R2
Osteoarthritis (OA) is a major articular degenerative disease characterized by local chronic inflammation and cartilage degeneration. Notoginsenoside R1 (NR1) is one of the bioactive compounds of Panax notoginseng with multiple pharmacological activities. In this study, we investigated the protective effect of NR1 on OA. Rat primary chondrocytes were treated with 10 ng/ml interleukin (IL)-1β to induce an OA-like phenotype in vitro followed by treatment with NR1. The results showed that the IL-1β-induced increased expression of tumor necrosis factor (TNF)-α, IL-6, and inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) was inhibited by NR1. In addition, NR1 downregulated the expression of matrix degrading enzymes and alleviated the degradation of extracellular matrix (ECM). Moreover, activation of the nuclear factor-erythroid 2-related factor-2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway was observed after NR1 treatment, which further inhibited the IL-1β-induced activation of the nuclear factor-κB (NF-κB) pathway. Knee OA was induced in rats in vivo by anterior cruciate ligament transaction (ACLT), and NR1 was then administered to rats by gavage. The results revealed that NR1 reduced cartilage degeneration and OA scores of the knee. In conclusion, the present study indicated that NR1 alleviates the inflammation of osteoarthritis by activating the Nrf2/HO-1 signaling pathway, indicating that NR1 may be a potential drug for OA therapy.
Notoginsenoside R1 (NGR1), the primary bioactive compound found in Panax notoginseng, is believed to have antihypertrophic and antiapoptotic properties, and has long been used to prevent and treat cardiovascular diseases. However, its potential role in prevention of diabetic cardiomyopathy remains unclear. The present study aimed to investigate the mechanism of NGR1 action in high glucose-induced cell injury. H9c2 cardiomyocytes were cultured in a high-glucose medium as an in-vitro model, and apoptotic cells were visualized using TUNEL staining. Expression of Nrf2 and HO-1 was measured using Western blotting or reverse transcription-quantitative PCR (RT-qPCR). The Nrf2 small interfering (si) RNA was transfected into cardiomyocytes using Opti-MEM containing Lipofectamine® RNAiMAX. NGR1 protected H9c2 cardiomyocytes from cell death, apoptosis and hypertrophy induced by high glucose concentration. Expression of auricular natriuretic peptide and brain natriuretic peptide was remarkably reduced in NGR1-treated H9C2 cells. Western blot analysis showed that high glucose concentration markedly inhibited AMPK, Nrf2 and HO-1, and this could be reversed by NGR1 treatment. However, the cardioprotective effect of NGR1 was attenuated by compound C, which reverses Nrf2 and HO-1 expression levels, suggesting that AMPK upregulates Nrf2 and HO-1 gene expression, protein synthesis and secretion. Transfection of H9C2 cells with Nrf2 siRNA markedly reduced the cardioprotective effect of NGR1 via reduced expression of HO-1. These results indicated that NGR1 attenuated high glucose-induced cell injury via AMPK/Nrf2 signaling and its downstream target, the HO-1 pathway. We conclude that the cardioprotective effects of NGR1 result from upregulation of AMPK/Nrf2 signaling and HO-1 expression in cardiomyocytes. Our findings suggest that NGR1 treatment might provide a novel therapy for diabetic cardiomyopathy.
Inflammation and apoptosis of vascular endothelial cells play a key role in the occurrence and development of atherosclerosis (AS), and the AMPK/mTOR/Nrf2 signaling pathway plays an important role in alleviating the symptoms of AS. Geniposide combined with notoginsenoside R1 (GN combination) is a patented supplement for the prevention and treatment of AS. It has been proven to improve blood lipid levels and inhibit the formation of AS plaques; however, it is still unclear whether GN combination can inhibit inflammation and apoptosis in AS by regulating the AMPK/mTOR/Nrf2 signaling pathway and its downstream signals. Our results confirmed that the GN combination could improve blood lipid levels and plaque formation in ApoE−/− mice fed with a high-fat diet (HFD), inhibit the secretion of serum inflammatory factors and oxidative stress factors. It also decreased the expression of pyrin domain containing protein 3 (NLRP3) inflammasome-related protein and Bax/Bcl2/caspase-3 pathway-related proteins. At the same time, the GN combination could also inhibit the H2O2-induced inflammatory response and apoptosis of human umbilical vein endothelial cells (HUVECs), which is mainly related to the activation of the AMPK/mTOR pathway by GN combination, which in turn induces the activation of Nrf2/HO-1 signal. In addition, the above phenomenon could be significantly reversed by dorsomorphin. Therefore, our experiments proved for the first time that the GN combination can effectively inhibit AS inflammation and apoptosis by activating the AMPK/mTOR/Nrf2 signaling pathway to inhibit the NLRP3 inflammasome and Bax/Bcl2/caspase-3 pathway.
Oleanolic acid
The results showed the enhanced activation and expression of Nrf2 as a result of treatment with OAO derivatives themselves and to less extent by their ASP conjugates, mainly in HepG2 cells. The association between cytotoxicity evaluated in our previous study and Nrf2 activation was observed. In this regard, compounds (18) with morpholide substituent at the C-17 position of OAO molecule and (12) with methyl ester substituent at the same position of OAO molecule to the most extent activated Nrf2 and subsequently cell cycle arrest at G2/M, leading to increased apoptosis and the number of resting HepG2 cells. The derivative of OAO (18) substituted with ASP (19) also affected Nrf2 activation and expression, but this effect was less pronounced in comparison with non-conjugated OAO. However, conjugation enhanced Nrf2 activation in normal THLE-2 cells.
These results confirmed our earlier suggestion that OAO derivatives conjugated with ASP have the potential for application in the liver cancer chemoprevention. OAO themselves, particularly OAO substituted with morpholide, may be considered therapeutic agents, which may support conventional treatment strategy. Further studies are required to confirm this suggestion.
Olive Leaf Extract OleuropeOriganum vulgare extract
The results revealed that the group supplied with the 0.1% OLE significantly exhibited a higher final body weight (FBW), weight gain (WG%), and specific growth rate (SGR) with a decreased feed conversion ratio (FCR) compared to the other groups (p < 0.05). An increase in immune response was also observed in the fish from this group, with higher lysosome activity, immunoglobulin (IgM), and respiratory burst than nonsupplemented fish, both before and after the A. hydrophila challenge (p < 0.05). Similarly, the supplementation of the 0.1% OLE also promoted the C. carpio’s digestive capacity pre- and post-challenge, presenting the highest activity of protease and alkaline phosphatase (p < 0.05). In addition, this dose of the OLE enhanced fish antioxidant capacity through an increase in the activity of superoxide dismutase (SOD) and glutathione peroxidase (GPx) and decreased hepatic lipid peroxidation end products (malondialdehyde—MDA), when compared to the control group, both pre- and post-infection (p < 0.05). Concomitantly with the superior immune response and antioxidant capacity, the fish fed the 0.1% OLE revealed the highest survival rate after the challenge with A. hydrophila (p < 0.05). A significant remarkable upregulation of the hepatic sod, Nrf2, and protein kinase C transcription levels was detected as a vital approach for the prevention of both oxidative stress and inflammation compared to the infected unsupplied control group (p < 0.05). Interestingly, HPLC and UPLC-ESI-MS/MS analyses recognized that oleuropein is the main constituent (20.4%) with other 45 compounds in addition to tentative identification of two new compounds, namely oleuroside-10-carboxylic acid (I) and demethyl oleuroside-10-carboxylic acid (II). These constituents may be responsible for the OLE exerted potential effects. To conclude, the OLE at a dose range of 0.66–0.83 g/kg w/w can be included in the C. carpio diet to improve the growth, antioxidant capacity, and immune response under normal health conditions along with regulating the infection-associated pro-inflammatory gene expressions, thus enhancing resistance against A. hydrophila.
Systemic lupus erythemathosus (SLE) is a chronic inflammatory and autoimmune disease which can affect multiple organ systems, without an effective and safe treatment. Olive leaf extracts are of special interest for their therapeutic effects. Oleuropein (OL) is the most abundant constituents of olive leaf extract and possesses many beneficial properties. In this study, we evaluated the effects of dietary OL and its new derivate, peracetylated oleuropein (Per-OL), in a pristane-induced SLE model. Mice received an injection of pristane or saline solution and were fed with experimental diets: enriched with OL and Per-OL. The levels of proinflammatory cytokines and markers were evaluated by enzyme-linked immunosorbent assay. The protein expressions of inducible nitric oxide synthase, microsomal prostaglandin E synthase 1, heme oxygenase (HO-1), nuclear factor E2-related factor 2 (Nrf2), mitogen-activated protein kinases (MAPKs), Janus kinase/signal transducer and activator of transcription (JAK/STAT), nuclear transcription factor-kappa B (NF-κB) and inflammasome nucleotide-binding domain, leucine-rich repeats-containing family, pyrin domain-containing-3 (NLRP3) pathways activation were determined in kidneys by Western blot. OL and Per-OL significantly reduced renal damage and decreased serum matrix metalloproteinase 3 and prostaglandine E2 kidneys levels.
Our findings indicate that Nrf2 and HO-1 antioxidant protein expressions were up-regulated in mice fed with OL and Per-OL diets, whereas the activation of JAK/STAT, MAPK, NF-κB and NLRP3 inflammasome pathways was significantly ameliorated. These results suggest that OL and Per-OL supplementation might provide a new alternative approach as a preventive/palliative treatment of nephritis in SLE management.
Olive leaf extract (OLE) has potential health benefits and protects against cytotoxicity in different organs. However, nothing has yet been reported on its potential to prevent cyclophosphamide (CP)-induced nephrotoxicity. This study investigated the possible protective effect of OLE on CP-induced kidney injury in rats, focusing on oxidative stress, inflammation, apoptosis and Nrf2/ARE/HO-1 signaling. Rats received 100 or 200 mg/kg body weight OLE for 15 days and a single injection of 150 mg/kg CP at day 16. CP induced kidney injury evidenced by the significantly increased serum creatinine and urea, and histopathological alterations, including glomerular atrophy, interstitial hemorrhage, dilated urinary space and necrosis.
CP-induced rats exhibited increased kidney lipid peroxidation, protein carbonyl, nitric oxide (NO) and pro-inflammatory cytokines, and up-regulated NF-κB, Bax, cytochrome c and caspase-3. OLE ameliorated kidney function markers and prevented CP-induced tissue damage. In addition, OLE significantly prevented oxidative stress, inflammation and apoptosis by enhancing the antioxidant defenses and Bcl-2 expression, and suppressing the pro-inflammatory and pro-apoptotic markers NF-κB, Bax, cytochrome c and caspase-3. OLE up-regulated Nrf2, HO-1 and NQO-1 expression in the kidney of CP-induced rats. In conclusion, OLE has a substantial protective role against CP-induced nephrotoxicity in rats by up-regulating the Nrf2/ARE/HO-1 signaling, enhancing the antioxidant activity and attenuating inflammation and apoptosis.
P-coumaric acid
In our work, we sought to study the potential of PCA in contradiction of ethanol induced hepatoxicity which linking with MAPKs, apoptosis, oxidative stress, and Nrf2 signaling. Foremost, we found that PCA could protect ethanol induced both L-02 and HepG2 hepatic cells by inhibiting cytotoxicity, ROS production, mitochondrial depolarization, and nuclear fragmentation. Also, in vivo experiments showed that the ethanol increasing the lipid markers (TBARS, CD) and depletes the antioxidants thereby increased phosphorylation of JNK, ERK, and p38 in rat liver tissues. Interestingly, PCA treatments inhibit ethanol exposed lipid markers and depletion of antioxidants, which directs the inhibition of MAPKs activation in rat liver tissues. We also noticed that the PCA protected ethanol induced apoptosis and liver markers by inhibiting the expression of Bax, caspases; AST, ALT, ALS, and LDH in liver tissue.
Overall, the ameliorative consequence of PCA on ethanol induced oxidative stress and apoptosis was achieved by suppressing the expression of CYP2E1 and overexpressing Nrf2 and its target protein HO-1 in rat liver tissue. As a result, PCA was marked to be an effective antioxidant with notable hepatoprotection by inhibiting MAPKs and apoptosis signaling via enhancing Nrf2 signaling.
Perilla frutescens extract
Perillaldehyde Inhibits AHR signaling and activates Nrf2 Antioxidant Pathway in Human Keratinocytes
The skin covers the outer surface of the body, so the epidermal keratinocytes within it are susceptible to reactive oxygen species (ROS) generated by environmental pollutants such as benzo(a)pyrene (BaP), a potent activator of aryl hydrocarbon receptor (AHR). Antioxidant activity is generally mediated by the nuclear factor-erythroid 2-related factor-2 (Nrf2) and heme oxygenase-1 (HO1) axis in human keratinocytes. Perillaldehyde is the main component of Perilla frutescens, which is a medicinal antioxidant herb traditionally consumed in East Asia. However, the effect of perillaldehyde on the AHR/ROS and/or Nrf2/HO1 pathways remains unknown. In human keratinocytes, we found that perillaldehyde (1) inhibited BaP-induced AHR activation and ROS production, (2) inhibited BaP/AHR-mediated release of the CCL2 chemokine, and (3) activated the Nrf2/HO1 antioxidant pathway. Perillaldehyde is thus potentially useful for managing inflammatory skin diseases or disorders related to oxidative stress.
The nuclear translocation of Nrf2 activates the up-stream regulator of HO-1 expression. Therefore, to verify whether PLE induces the translocation of Nrf2 to the nucleus, nuclear fractions were extracted for Western Blot analysis. As shown in Fig. 8a and b, nuclear fractions of rat livers showed that Nrf2 expression in the PLE-pretreated group, specifically with 1,000 mg/kg b.w. of PLE, significantly increased compared with the group with t-BHP treatment alone.
The nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element (ARE) pathway is a cellular defense system against oxidative stress. activation of this pathway increases expression of antioxidant enzymes. Epidemiological studies have demonstrated that the consumption of fruits and vegetables is associated with reduced risk of contracting a variety of human diseases. The aim of this study is to find Nrf2-ARE activators in dietary fruits and vegetables. We first attempted to compare the potency of ARE activation in six fruit and six vegetables extracts. Green perilla (Perilla frutescens var. crispa f. viridis) extract exhibited high ARE activity. We isolated the active fraction from green perilla extract through bioactivity-guided fractionation. Based on nuclear magnetic resonance and mass spectrometric analysis, the active ingredient responsible for the ARE activity was identified as 2′,3′-dihydroxy-4′,6′-dimethoxychalcone (DDC). DDC induced the expression of antioxidant enzymes, such as γ-glutamylcysteine synthetase (γ-GCS), NAD(P)H: quinone oxidoreductase-1 (NQO1), and heme oxygenase-1. DDC inhibited the formation of intracellular reactive oxygen species and the cytotoxicity induced by 6-hydroxydopamine. Inhibition of the p38 mitogen-activated protein kinase pathway abolished ARE activation, the induction of γ-GCS and NQO1, and the cytoprotective effect brought about by DDC. Thus, this study demonstrated that DDC contained in green perilla enhanced cellular resistance to oxidative damage through activation of the Nrf2-ARE pathway.
Petroselinum crispum extract
Parsley, a common vegetable, is widely used as a condiment because its flavor. However, its biological functionality remains largely ignored. In this study, the antioxidant activity-guided separation method was used to obtain four characteristic 7-O-flavonoid glycosides from parsley. An excessive fatigue mouse model was used to evaluate the anti-fatigue effects of the different parsley extracts. The results showed that parsley flavonoids could significantly improve fatigue related physiological indicators and morphology of the gastrocnemius muscle. Western blotting indicated that parsley flavonoids could regulate oxidative stress and exert an anti-fatigue effect by regulating the Keap1/Nrf2 and AMPK/PGC-1α pathways. Furthermore, the intake of flavonoids stimulated the enrichment of probiotics and short chain fatty acid producing microbiota, which likely participate in the body’s energy metabolism and regulate fatigue. The present findings expand the conventional understanding of parsley as a seasoning food, proving that it has the potential for further development as a functional food.
phenethyl isothiocyanate
Phenethyl Isothiocyanate, a Dual activator of Transcription Factors Nrf2 and HSF1
Cruciferous vegetables are rich sources of glucosinolates which are the biogenic precursor molecules of isothiocyanates (ITCs). The relationship between the consumption of cruciferous vegetables and chemoprotection has been widely documented in epidemiological studies. Phenethyl isothiocyanate (PEITC) occurs as its glucosinolate precursor gluconasturtiin in the cruciferous vegetable watercress (Nasturtium officinale). PEITC has multiple biological effects, including activation of cytoprotective pathways, such as those mediated by the transcription factor nuclear factor erythroid 2 p45‐related factor 2 (Nrf2) and the transcription factor heat shock factor 1 (HSF1), and can cause changes in the epigenome. However, at high concentrations, PEITC leads to accumulation of reactive oxygen species and cytoskeletal changes, resulting in cytotoxicity. Underlying these activities is the sulfhydryl reactivity of PEITC with cysteine residues in its protein targets. This chemical reactivity highlights the critical importance of the dose of PEITC for achieving on‐target selectivity, which should be carefully considered in the design of future clinical trials.
Phloretin
Our findings reveal that phloretin significantly decreased the levels of intracellular reactive oxygen species (ROS) and malondialdehyde (MDA), increased superoxide dismutase (SOD) and glutathione peroxidase-1 (Gpx-1) activity, and restored the loss of mitochondrial membrane potential (MMP). Next, whole transcriptome analysis was performed using RNA-Seq The results indicated more than 3000 differentially expressed genes (DEGs). Gene Ontology analysis revealed that the DEGs were categorized functionally, mainly by the biological processes, cell metabolism, and cellular response to chemical stimulus. The Kyoto Encyclopedia of Genes and Genomes indicated that they were mainly enriched in cAMP, apoptosis, and cytoskeletal regulation signaling pathways.
Furthermore, on the basis of the results of RNA-Seq and Western blotting, our study verified that phloretin upregulated the expression of p-Nrf2 and HO-1 by promoting the phosphorylation of AMPK at Thr172 through activation of liver kinase B1. In conclusion, phloretin attenuates PA-induced oxidative stress in HUVECs via the AMPK/Nrf2 antioxidative pathway.
Piper methysticum
Objective To compare the toxicity flavokawains A and B (FKA, FKB) and monitor the resulting transcriptional responses and cellular adaptation in the human hepatocyte cell line, HepG2. Materials and methods HepG2 were treated with 2-100 μM FKA or FKB for 24-48 h. Cellular viability was measured with calcein-AM and changes in signalling and gene expression were monitored by luciferase reporter assay, real-time PCR and Western blot of both total and nuclear protein extracts. To test for subsequent resistance to oxidative stress, cells were pretreated with 50 μM FKA, 10 μM FKB or 10 μM sulphoraphane (SFN) for 24 h, followed by 0.4-2.8 mM H2O2 for 48 h, and then viability was assessed. Results FKA (≤100 μM) was not toxic to HepG2, whereas FKB caused significant cell death (IC50=23.2 ± 0.8 μM). Both flavokawains activated Nrf2, increasing HMOX1 and GCLC expression and enhancing total glutathione levels over 2-fold (p < 0.05). FKA and FKB also activated HSF1, increasing HSPA1A and DNAJA4 expression. Also, flavokawain pretreatment mitigated cell death after a subsequent challenge with H2O2, with FKA being more effective than FKB, and similar to SFN. Conclusions Flavokawains promote an adaptive cellular response that protects hepatocytes against oxidative stress. We propose that FKA has potential as a chemopreventative or chemotherapeutic agent.
Polygonum cuspidatum (Japanese knotweed)
Results: PCE significantly alleviated liver lipid accumulation in fructose-fed rats with metabolic syndrome. It also inhibited Keap1, activated Nrf2 antioxidant pathway, resulting in the suppression of oxidative stress, evidenced by reducing hydrogen peroxide (H2O2), malondialdehyde (MDA) and hydroxy radical (OH•) levels, and increasing glutathione (GSH)/oxidized glutathione (GSSG) ratio as well as superoxidase dismutase (SOD) and catalase (CAT) activity in the liver of fructose-fed rats. Additionally, PCE up-regulated peroxisome proliferator activated receptor-α (PPAR-α), and down-regulated sterol regulatory element binging protein 1 (SREBP-1), fatty acid synthetase (FAS) and stearoyl-CoA desaturase-1 (SCD-1) in this animal model, being consistent with its reduction of triglyceride (TG) levels.
Conclusion: These results demonstrate that PCE reduces oxidative stress, and prevent lipid accumulation in the liver of fructose-fed rats possibly by targeting the Keap1/Nrf2 pathway. PCE may be a promising therapeutic strategy for fructose-associated liver lipid accumulation.
Advances for pharmacological activities of Polygonum cuspidatum
In the ovalbumin-induced asthmatic mice model, polydatin could increase the activity of SOD and CAT in bronchoalveolar lavage fluid and decrease the content of ROS and malondialdehyde (MDA). The mechanism might be related to activating the p38 MAPK/nuclear factor E2-related factor 2 (Nrf2) signal pathway (Zhao, Jiang et al. 2018; Zeng et al. 2019).
Researchers found that polydatin could also reduce the ROS content in the fat tissue of the retrobulbar of Graves’ orbitopathy mice model by stimulating the Kelch like-ECH-associated protein 1 (Keap1)/Nrf2 pathway in orbital fibroblasts (Li et al. 2020). The stimulation of PC on Keap1/Nrf2 could also reduce the ROS level in the liver tissue of rats with fructose-induced liver injury and inhibit fatty liver degeneration (Zhao, Yu et al. 2018). Figure 2 depicts the primary mechanisms of PC’s antioxidant effect. Studies on the antioxidant effects of PC and its extracts are listed in Table 2.
Pomegranate extract
Pomegranate (Punica granatum L.) phenolic compounds may prevent oxidative stress-induced neurodegenerative diseases. The present study investigated cytoprotective effects of pomegranate wine extracts and their phenolic compounds in neuroblastoma cells SH-SY5Y. The extracts significantly activated nuclear factor erythroid 2-related factor 2 (Nrf2; ≤2.6-fold) and inhibited nuclear factor κB (NF-κB) nuclear translocation (≥26% activity) without cytotoxic effects. Moreover, pomegranate wine extract time-dependently elevated heme oxygenase-1 (HO-1) expression (≤2.9-fold) and promoted superoxide dismutase (SOD) activity (≤1.4-fold), while it had no significant effect on glutathione peroxidase (GPx) activity. The main phenolic components of the extracts, gallic acid, ellagic acid, and punicalagin also led to a significant inhibition of NF-κB and the activation of Nrf2 translocation and subsequent increase of HO-1 expression and SOD activity, dependent on concentration and incubation time. Thus, pomegranate wine and its phenolic components may have a protective effect on neuronal cells by triggering Nrf2 and inhibiting NF-κB signaling.
Studies have shown a relationship between pomegranate peel extract supplementation and protection against myocardial tissue failure [25]. As evidenced in isoproterenol-induced myocardial infarction (MI) in Wistar rats, the Punica granatum L. peel extract attenuated electrocardiographic changes, myocardial hypertrophy, and lipid peroxidation, and decreased the serum markers of MI. The maximum result was provided with the highest analyzed dosage, 200 mg/kg BW. The upregulation of myocardial expression of endothelial nitric oxide synthase (eNOS), activating nitric-oxide-mediated Nrf2, was concluded to be responsible for the cardioprotection of extract treatment [67]. Similarly, Punica granatum L. peel tended to protect the myocardium against toxic agents, demonstrated by the mitigation of ECG disturbances and biochemical parameters [63,65]. Contrary to these outcomes, we did not observe a marked alternation of cTnI level in the rats treated with ethanolic extract. Interestingly, a prominent troponin elevation was detected in healthy rats receiving the phenolic extract. Therefore, it may be concluded that an 8-week EPP exposure might not be enough to moderate the chronic progression of heart failure.
Potato extract
In this study, it is aimed to investigate the antioxidant mechanism of new extracts from potatoes. Four pigments, namely, Petunin, Paeonin, Malvidin and Pelargonidin, were extracted from potatoes by high performance liquid chromatography (HPLC). Our results showed that the cellular morphology and cell viability were significantly altered in gastric mucosal epithelial cells (GES-1) treated with different hydrogen peroxide (H2O2) concentrations over time (P < 0.05). Paeonin presented the strongest anti-oxidative effects on H2O2-treated cells, in both a dose- and time-dependent manner, determined by ARE-luciferase activity and HO-1 mRNA expression. After pre-treatment with Paeonin in H2O2-exposed cells, Keap1, Nrf2, HO-1 and NQO1 protein expressions were remarkably up-regulated. Furthermore, immunostaining of Nrf2 expression was obviously elevated in the H2O2 + Paeonin group over time. The GSH content in the H2O2 + Paeonin group was notably lower than that in the H2O2 + Paeonin + GSK690693 group. Paeonin promoted cell cycle with augmented Cyclin D1 and p27 protein expressions. Moreover, Paeonin suppressed apoptosis with increased Bcl2, total Caspase3 and total Caspase8 protein expressions and decreased Bax, p-Caspase3 and p-Caspase8 protein expression in H2O2-treated cells. These results suggested that Paeonin might exert an anti-oxidative role by activating Nrf2 signaling pathway with the changes of cell cycle and apoptosis.
Procyanidin B2
Cancer is one of the leading causes of death worldwide. Chemotherapy and radiation therapy are currently providing the basis for cancer therapies, although both are associated with significant side effects. Thus, cancer prevention through dietary modifications has been receiving growing interest. The potential of selected flavonoids in reducing carcinogen-induced reactive oxygen species (ROS) and DNA damage through the activation of nuclear factor erythroid 2 p45 (NF-E2)-related factor (Nrf2)/antioxidant response element (ARE) pathway was studied in vitro. Dose-dependent effects of pre-incubated flavonoids on pro-carcinogen 4-[(acetoxymethyl)nitrosamino]-1-(3-pyridyl)-1-butanone (NNKAc)-induced ROS and DNA damage in human bronchial epithelial cells were studied in comparison to non-flavonoids. The most effective flavonoids were assessed for the activation of Nrf2/ARE pathway. Genistein, procyanidin B2 (PCB2), and quercetin significantly suppressed the NNKAc-induced ROS and DNA damage. Quercetin significantly upregulated the phosphorylated protein kinase B/Akt. PCB2 significantly upregulated the activation of Nrf2 and Akt through phosphorylation. Genistein and PCB2 significantly upregulated the phospho-Nrf2 nuclear translocation and catalase activity. In summary, genistein and PCB2 reduced the NNKAc-induced ROS and DNA damage through the activation of Nrf2. Further studies are required to understand the role of dietary flavonoids on the regulation of the Nrf2/ARE pathway in relation to carcinogenesis.
protocatechuic acid
Protocatechuic acid (PCA) is a main metabolite of anthocyanins, whose daily intake is much higher than that of other polyphenols. PCA has biological effects, e.g., it induces the antioxidant/detoxifying enzyme gene expression. This study was aimed at defining the molecular mechanism responsible for PCA-induced over-expression of glutathione (GSH) peroxidase (GPx) and GSH reductase (GR) in J774 A.1 macrophages. New evidence is provided that PCA increases GPx and GR expression by inducing C-JUN NH(2)-terminal kinase (JNK)-mediated phosphorylation of Nuclear factor erythroid 2 (NF-E2)-related factor 2 (Nrf2). RNA and proteins were extracted from cells treated with PCA (25 μM) for different time points. Quantitative real-time polymerase chain reaction and immunoblotting analyses showed a rapid increase in mRNA (>60%) and protein (>50%) for both the enzymes. This was preceded by the up-regulation of Nrf2, in terms of mRNA and protein, and by its significant activation as assessed by increased Nrf2 phosphorylation and nuclear translocation (+60%). By using specific kinase inhibitors and detecting the activated form, we showed that JNK was the main upstream kinase responsible for Nrf2 activation. Convincing evidence is provided of a causal link between PCA-induced Nrf2 activation and increased enzyme expression. By silencing Nrf2 and using a JNK inhibitor, enzyme enhancement was counteracted. Finally, with the ChIP assay, we demonstrated that PCA-activated Nrf2 specifically bound ARE sequences in enzyme gene promoters. Our study demonstrates for the first time that PCA improves the macrophage endogenous antioxidant potential by a mechanism in which JNK-mediated Nrf2 activation plays an essential role. This knowledge could contribute to novel diet-based approaches aimed at counteracting oxidative injury by reinforcing endogenous defences.
Caffeic acid and protocatechuic acid modulate Nrf2 and inhibit Ehrlich ascites carcinomas in mice
FRAP and DPPH radical scavenging assays showed that caffeic acid and protocatechuic acid were more potent compared with cinnamic acid and benzoic acid. Luciferase complementation reporter assays identified caffeic acid and protocatechuic acid as the activators of Nrf2. Both caffeic acid and protocatechuic acid upregulated the expression of Nrf2 target genes heme oxygenase-1 (HO-1), glutamate-cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase modifier subunit (GCLM) and the activity of NAD(P)H:quinone oxidoreductase 1 (NQO1) when tested on HCT-116 cells using a cell-based assay system at 9 h. In addition, intraperitoneal administration of caffeic acid and protocatechuic acid to Ehrlich ascites carcinoma bearing mice suppressed tumor growth and angiogenesis. Caffeic acid and protocatechuic acid can modulate Nrf2 and inhibit Ehrlich ascites carcinoma cells.
Purple sweet potato extract
Anthocyanins of the purple sweet potato exhibit antioxidant and hepatoprotective activities via a multitude of biochemical mechanisms. However, the signaling pathways involved in the actions of anthocyanin-induced antioxidant enzymes against chronic liver injury are not fully understood. We examined whether an anthocyanin fraction (AF) from purple sweet potato may prevent dimethylnitrosamine (DMN)-induced liver injury by inducing antioxidants via nuclear erythroid 2-related factor 2 (Nrf2) pathways and by reducing inflammation. Treatment with AF attenuated the DMN-induced increased serum alanine aminotransferase and aspartate aminotransferase activities. It also prevented the formation of hepatic malondialdehyde and the depletion of glutathione and maintained normal glutathione-S-transferase (GST) activity in the livers of DMN-intoxicated rats. Furthermore, AF increased the expression of Nrf2, NADPH:quinine oxidoreductase-1, heme oxygenase-1, and GSTα, which were reduced by DMN, and decreased the expression of cyclooxygenase-2 and inducible nitric oxide synthase. An increase in the nuclear translocation of nuclear factor kappa B (NF-κB) was observed in the DMN-induced liver injury group, but AF inhibited this translocation. Taken together, these results demonstrate that AF increases the expression of antioxidant enzymes and Nrf2 and at the same time decreases the expression of inflammatory mediators in DMN-induced liver injury. These data imply that AF induces antioxidant defense via the Nrf2 pathway and reduces inflammation via NF-κB inhibition.
Quercetin
Age-related macular degeneration (AMD) represents a major reason for blindness in the elderly population. Oxidative stress is a predominant factor in the pathology of AMD. We previously evaluated the effects of phospholipid complex of quercetin (Q-PC) on oxidative injury in ARPE-19 cells, but the underlying mechanisms are not fully understood. Herein, the solid dispersion of quercetin-PC (Q-SD) was prepared with solubility being 235.54 μg/mL in water and 2.3×104 μg/mL in chloroform, which were significantly higher than that of quercetin (QT) and Q-PC. Q-SD also exhibited a considerably higher dissolution rate than QT and Q-PC. Additionally, Q-SD had Cmax of 4.143 μg/mL and AUC of 12.015 μg·h/mL in rats, suggesting better bioavailability than QT and Q-PC. Then, a mouse model of dry AMD (Nrf2 wild-type (WT) and Nrf2 knockout (KO)) was established for evaluating the effects of Q-SD in vivo. Q-SD more potently reduced retinal pigment epithelium sediments and Bruch’s membrane thickness than QT and Q-PC at 200 mg/kg in Nrf2 WT mice and did not work in Nrf2 KO mice at the same dosage. Additionally, Q-SD significantly decreased ROS and MDA contents and restored SOD, GSH-PX, and CAT activities of serum and retinal tissues in Nrf2 WT mice, but not in Nrf2 KO mice. Furthermore, Q-SD more potently increased Nrf2 mRNA expression and stimulated its nuclear translocation in retinal tissues of Nrf2 WT mice. Q-SD significantly increased the expression of Nrf2 target genes HO-1, HQO-1, and GCL of retinal tissues in Nrf2 WT mice, not in Nrf2 KO mice. Altogether, Q-SD had improved physicochemical and pharmacokinetic properties compared to QT and Q-PC and exhibited more potent protective effects on retina oxidative injury in vivo. These effects were associated with activation of Nrf2 signaling and upregulation of antioxidant enzymes.
Modulation of Nrf2 by quercetin in doxorubicin-treated rats
Doxorubicin (DOXO), a potent and widely used chemotherapeutic agent, causes irreversible heart failure by increasing oxidative stress, which limits its clinical utility. Nuclear factor erythroid-derived 2 -like 2 (Nrf2) is a prominent central regulator of cellular impenetrable to oxidants. The purpose of the study is to assess the ameliorative outcome of quercetin in cardiomyopathic rats induced by doxorubicin. Cardiomyopathy was produced in rats by single intraperitoneal weekly with DOXO (2 mg/kg) for 4 weeks. The rats were divided into five groups: (I) control group; (II) DOXO (2 mg/kg, i.p.) group; (III–V) DOXO + quercetin (10 mg/kg, 25 mg/kg and 50 mg/kg, orally), and were treated for 7 weeks. At the end of the treatment duration, cardiac function and biochemical parameters were assessed. Quercetin (10 mg/kg, 25 mg/kg and 50 mg/kg, orally) treatment reduced the raised blood pressure (BP) and left ventricular dysfunction. Withal, it prevented the rise in CKMB and LDH, suggesting the effect of quercetin in the maintaining the integrity of the cell membrane Besides, it also prevented the alteration in electrolyte levels, the activity of ATPase, and antioxidant status. Quercetin increased Nrf2 mRNA expression and reduced histological abnormalities compared 1to the DOXO control group. In conclusion, quercetin protected against DOXO- induced cardiomyopathy, by increasing expression of Nrf2, and thereby increasing antioxidant defense and restoring biochemical and histological abnormalities.
Nuclear factor-erythroid 2 related factor (Nrf2) is involved in cell defense and survival against endogenous and exogenous stress. Constitutively active Nrf2 in malignant cells increases the expression of cytoprotective genes and consequently, enhances proliferation via metabolic reprograming and inhibition of apoptosis (Leinonem, Advances in cancer Research, 2014). Nrf2 is persistently activated in many human tumors, including acute myelogenous leukemia thus, inhibition of Nrf2 activity may be a promising strategy for leukemia therapy. Flavonoids, present in vegetables, fruit and propolis, might exert antitumoral effects through induction of apoptosis and chromatin remodeling (Link, Biochem Pharmacol., 2010). A previous study from our group showed that Quercetin (Qu), a natural polyphenolic flavonoid compound, induced apoptosis, partly due to its DNA demethylating activity, through HDAC inhibition and by the enrichment of H3ac and H4ac in the promoter regions of genes involved in the apoptosis pathway, leading to their transcription activation (Alvarez, Clinical epigenetics 2018). In the present study, we evaluated the effect of Qu as a modulator of Nrf2.
This study was performed in vivo in human xenograft acute myeloid leukemia (AML) models, and in vitro using leukemia cell lines. Qu treatment (50 µM Qu) for 48h downregulated HDAC4, Nrf2 and p-Nrf2 at protein levels (p<0.05; p<0.005; p<0.005 respectively). Imaging Flow Cytometry (AMNIS, ImageStream ISX mkIITM) and Confocal Microscopy evidenced a decrease in Nrf2 nuclear localization. Furthermore, combined treatment with the proteasome inhibitor MG132 prevented degradation of Nrf2, indicating that treatment increased proteasomal degradation. Loss of Nrf2 decreases HDAC4, a redox sensitive histone deacetylase, resulting in an increased expression of miR-1 and miR-206 (Singh, J Clin Invest. 2013). Herein, expression profile of 84 miRNAs (Apoptosis miRNA PCR array) were performed in samples from human xenograft model. Treatment up-regulated the expression profile of 5% (n=4) of the 84 miRNAs evaluated, corresponding exclusively to miRNAs that target anti-apoptotic genes and to miRNAs that have been demonstrated to have pro-apoptotic functions.
Furthermore, expression levels of miR-1, miR-133a/b, which target anti-apoptotic genes and miR-206, a pro apoptotic miR, were validated in xenograft model samples, resulting in a significant up-regulation of the expression levels in treated animals compared to controls (p<0.05). In addition, lentiviral sh down regulation of Nrf2, led to an increased apoptosis, decreased cell survival and an up regulation of miRNA 206 expression in Qu treated cells. In summary, Qu might induce programmed cell death in part, by decreasing Nrf2 nuclear localization, by inducing Nrf2 proteasomal degradation and down regulation of HDAC4 which led to up-regulation of pro apoptotic miRNAs.
Raphanus sativus
Our findings show that BRE significantly ameliorated LPS-induced nitric oxide (NO) release and production of pro-inflammatory cytokines, such as IL-1β, IL-6, tumor necrosis factor (TNF)-α, and prostaglandin E2. The levels of cyclooxygenase (COX)-2 and inducible NO synthase (iNOS) in LPS-stimulated RAW 264.7 cells were found to be suppressed by BRE. Further, BRE significantly suppressed the LPS-induced expression of mRNAs encoding COX-2, iNOS, IL-1β, IL-6, and TNF-α in a concentration-dependent manner. BRE treatment significantly inhibited Janus kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3) phosphorylation in IL-6- and LPS-treated RAW 264.7 cells. In addition, BRE decreased the levels of phosphorylated extracellular signal-regulated protein kinases and c-Jun N-terminal kinase under the same conditions. Moreover, BRE induced high nuclear factor erythroid 2-related factor 2 (Nrf2) levels and its target gene heme oxygenase 1 (HO-1) in the absence of LPS. These data demonstrate that BRE may be beneficial for treating inflammation through selective immunomodulatory effects, which may be mediated by inhibition of the STAT3/JAK2 and activation of the Nrf2/HO-1 signal transduction pathways.
These results are similar to those previously reported on the association between oxidative stress and apoptosis. Among several antioxidant mechanisms, Nrf2/HO-1 signaling plays a vital role in cell migration, growth, and death [48]. The pathway of Nrf2 is a key sensor that protects cells from oxidative stress by inducing cytoprotective gene transcription and is linked to cytoplasmic Keap1 in steady state. At this time, when ROS is excessively generated by oxidative stress, Keap1 releases Nrf2 and then migrates to the nucleus where it binds to AREs associated with other transcription factors and helper proteins. It induces transcription of several antioxidant genes, such as NAD(P)H:quinone oxidoreductase 1 (NQO1), HO-1, glutamate-cysteine ligasecatalytic subunit (GCLC), CAT, and SOD [49,50]. In addition, there are an increasing number of studies that can support Nrf2, the antioxidant key modulator, as an important factor in improving liver damage [51,52]. It is known that Nrf2 activates HO-1 expression, and upregulates the apoptosis inhibitor BCL-2, and on the contrary, downregulates the expression of BAX, c-Caspase3, and c-Caspase9 [53,54].
Studies indicate that oxidative stress is improved and apoptosis is suppressed by Nrf2, a multi-signaling pathway modulator [55]. Homing et al. [56] confirmed that the hepatoprotective effect of Lico A against acetaminophen-induced hepatotoxicity was via the regulation of Nrf2 and c-Caspase3, and the knockout of hepatocyte-specific JNK can alleviate mitochondrial oxidative stress and reduce apoptosis and liver damage [57]. Therefore, our radish and turnip extracts are also assumed to regulate oxidative stress and apoptosis by activating the Nrf2 factor. This effect may be attributed to glucosinolate, a compound in Brassicaceae. Glucosinolate is a sulfur-containing substance mainly found in the Brassica family, and has been reported to possess various physiological effects, such as anti-inflammatory and anticancer activities [58,59]. Isothiocyanate, a secondary hydrolysate formed by glucosinolate hydrolysis, is a biologically active compound with anticarcinogenic [60] and antifungal [61] properties. In particular, it regulates the Keap1/Nrf2/ARE pathway and promotes antioxidant and apoptotic effects in cancer cells [58].
Red wine extract
Our results proved that combined ethanolic extract of Vitis vinifera and Silymarin restored normal renal and cardiac histomorphology. Significant improvement of Creatinine, BUN, lipid profile and hs-CRP cardiac and renal biochemical indicators confirmed our results. Moreover, significant elevation of mRNA expression levels of Nrf2 proved that combined Vitis vinifera and Silymarin action was directly related to the redox-sensitive regulator pathway.
We concluded that synergistic therapeutic effect of Vitis vinifera extract and Silymarin on experimental cardiorenal injury model owes principally to promoting activation of the Keap1/Nrf2 signaling pathway.
Acute liver injury was induced by a single intraperitoneal administration of CCl4 (1 mL/kg). VCEC was administered orally for three consecutive days at various concentrations (100 and 200 mg/kg) prior to CCl4 injection. The extent of liver injury and the protective effects of VCEC were evaluated by biochemical analysis and histopathological studies. oxidative stress was evaluated by measuring malondialdehyde (MDA) and glutathione (GSH) levels and Western blotting. VCEC treatment significantly reduced serum transaminase levels (AST and ALT), tumor necrosis factor-α (TNF-α), and reactive oxygen species (ROS). CCl4- induced apoptosis was inhibited by VCEC treatment by reducing cleaved caspase-3 and Bcl2-associated X protein (Bax).
VCEC-treated mice significantly restored cytochrome P450 2E1, nuclear factor erythroid 2-related factor 2 (Nrf2), and heme oxygenase-1 (HO-1) expression in CCl4-treated mice. In addition, VCEC downregulated overexpression of proinflammatory cytokines and hepatic nuclear factor kappa B (NF-κB) and inhibited CCl4-mediated apoptosis. Collectively, VCEC exhibited synergistic protective effects against liver injury through its antioxidant, anti-inflammatory, and antiapoptotic ability against oxidative stress, inflammation, and apoptosis. Therefore, VCEC appears promising as a potential therapeutic agent for CCl4-induced acute liver injury in mice.
Rehmapicrogenin
In vivo, rehmapicrogenin treatment significantly attenuated the pathological changes in the kidney induced by ADR; rescued weight, serum creatinine (Scr), blood urea nitrogen (BUN) and urine albumin (U-ALB) levels; reduced reactive oxygen species (ROS) accumulation; and decreased oxidative stress, the apoptosis rate, and cell survival in ADR-treated mice. Importantly, both in vivo and in vitro experimental results demonstrated that rehmapicrogenin regulates the Nrf2/ARE signalling pathway, the most important pathway for oxidative stress. Rehmapicrogenin attenuated ADR-induced kidney damage by reducing oxidative stress through the oestrogen receptor pathway. Moreover, after treatment with ICI 182780 (the oestrogen receptor-nonspecific antagonist Faslodex), the improvement induced by rehmapicrogenin was significantly reversed. In conclusion, rehmapicrogenin attenuates kidney damage by reducing inflammatory factor release through the oestrogen signalling pathway.
Resveratrol
The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway
Resveratrol is a natural polyphenol derived from grapes, berries, red wine, peanuts amongst other fruits and nuts. Beneficial effects such as anti-inflammatory, antioxidant, hepatoprotective, neuroprotective, cardioprotective, renoprotective, anti-obesity, anti-diabetic, and anti-cancer of resveratrol have been demonstrated by preclinical and clinical research. A possibility is that these therapeutical effects are associated with modulation of the Nrf2 signaling pathway in the following way: resveratrol may potentiate Nrf2 signaling through blockage of Keap1, by means of changing the Nrf2 mediators, its expression and its nuclear translocation. This article reviews the evidence of the Nrf2 modulating hypothesis as a possible molecular mechanism underlying the medicinal properties of resveratrol.
Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury
Results. Resveratrol improved renal function, proteinuria, histological changes and inflammation in aging mice. Also, expression of Nrf2-HO-1-NOQ-1 signaling and SIRT1-AMPK-PGC-1α signaling was increased in the RSV group. Transfection with Nrf2 and SIRT1 siRNA prevented resveratrol-induced anti-oxidative effect in HK2 cells in media treated with H2O2.
Conclusions. activation of the Nrf2 and SIRT1 signaling pathways ameliorated oxidative stress and mitochondrial dysfunction. Pharmacological targeting of Nrf2 signaling molecules may reduce the pathologic changes of aging in the kidney.
Resveratrol mediates its anti-cancer effects by Nrf2 signaling pathway activation
Resveratrol is a natural compound that can activate the Nrf2 transcription factor. Nfr2 translocates to the nucleus and induces antioxidant gene expression. In different cell lines, resveratrol can increase apoptosis and inhibit the proliferation of cancer cells.
We found that resveratrol shows efficacy for the treatment of cancer, but due to high controversy on the Nrf2 signaling pathway and mechanisms of resveratrol action, additional studies should be conducted to better characterize its mode-of-action in cancer.
Retinoic acid
Isothiocyanates and phenolic antioxidants can prevent cancer through activation of Nrf2 (NF-E2 p45-related factor 2), a transcription factor that controls expression of cytoprotective genes through the antioxidant response element (ARE) enhancer. Using a human mammary MCF7-derived AREc32 reporter cell line, we now report that all-trans retinoic acid (ATRA), and other retinoic acid receptor alpha (RARα) agonists, markedly reduces the ability of Nrf2 to mediate induction of ARE-driven genes by cancer chemopreventive agents including the metabolite of butylated hydroxyanisole, tert-butylhydroquinone (tBHQ). The basal and tBHQ-inducible expression of aldo-keto reductase (AKR) AKR1C1 and AKR1C2 genes, which are regulated by Nrf2, was also repressed by ATRA in AREc32 cells. Antagonists of RARα augmented induction of ARE-driven gene expression by tBHQ, as did knockdown of RARα by using RNAi. The expression of the ARE-gene battery was increased in the small intestine of mice fed on a vitamin A-deficient diet, and this increase was repressed by administration of ATRA. By contrast, in the small intestine of Nrf2 null mice, the expression of ARE-driven genes was not affected by vitamin A status. In MCF7 cells, ATRA did not block the nuclear accumulation of Nrf2 but reduced the binding of Nrf2 to the ARE enhancer as a consequence of forming a complex with RARα. These data suggest that cross-talk between Nrf2 and RARα could markedly influence the sensitivity of cells to electrophiles and oxidative stressors and, as a consequence, to carcinogenesis.
The transcription factor Nrf2 (NFE2L2) is a pivotal activator of genes encoding cytoprotective and detoxifying enzymes that limit the action of cytotoxic therapies in cancer. Nrf2 acts by binding antioxidant response elements (ARE) in its target genes, but there is relatively limited knowledge about how it is negatively controlled. Here, we report that retinoic X receptor alpha (RXRα) is a hitherto unrecognized repressor of Nrf2. RNAi-mediated knockdown of RXRα increased basal ARE-driven gene expression and induction of ARE-driven genes by the Nrf2 activator tert-butylhydroquinone (tBHQ). Conversely, overexpression of RXRα decreased ARE-driven gene expression. Biochemical investigations showed that RXRα interacts physically with Nrf2 in cancer cells and in murine small intestine and liver tissues. Furthermore, RXRα bound to ARE sequences in the promoters of Nrf2-regulated genes. RXRα loading onto AREs was concomitant with the presence of Nrf2, supporting the hypothesis that a direct interaction between the two proteins on gene promoters accounts for the antagonism of ARE-driven gene expression. Mutation analyses revealed that interaction between the two transcription factors involves the DNA-binding domain of RXRα and a region comprising amino acids 209-316 in human Nrf2 that had not been defined functionally, but that we now designate as the Nrf2-ECH homology (Neh) 7 domain. In non–small cell lung cancer cells where Nrf2 levels are elevated, RXRα expression downregulated Nrf2 and sensitized cells to the cytotoxic effects of therapeutic drugs. In summary, our findings show that RXRα diminishes cytoprotection by Nrf2 by binding directly to the newly defined Neh7 domain in Nrf2.
Nrf2 as a determinant of cellular resistance in retinoic acid cytotoxicity
Clinical use of retinoic acids (RA) is hindered by toxicity possibly related to oxidative stress. Recently, RA at relatively low concentrations was shown to inhibit Nrf2 and the expression of its target antioxidative genes. This raises the possibility that RA toxicity may result from cellular inability to cope with resultant oxidative stress. Using in vitro cell and in vivo mouse models, we report that RA, specifically all-trans-RA (atRA) at concentrations implicated in toxicity, can activate Nrf2 and induce Nrf2 target genes, particularly the subunits of the rate-limiting enzyme of glutathione biosynthesis, glutamate cysteine ligase (GCLM/GCLC). RNA interference-mediated silencing of Nrf2, but not of retinoid X receptor-α and-β, reduced basal and atRA-induced GCLM/GCLC gene expression.
Moreover, RA increased nuclear accumulation of Nrf2, antioxidant response element (ARE) reporter activity, and Nrf2 occupancy at AREs. 4-Hydroxynonenal, a lipid peroxidation product, was increased by RA. Inhibition of MEK1/ERK mitogen-activated protein kinases significantly suppressed atRA-induced Nrf2 activation and ARE-regulated gene expression, reducing cell resistance against toxic concentrations of RA. Nrf2-silenced cells were vulnerable to atRA-induced mitochondrial toxicity and apoptosis. In conclusion, toxic RA activates Nrf2, thereby triggering an adaptive response against the resultant oxidative stress. Nrf2 enhancement as a therapeutic target of retinoid toxicity awaits further investigation.
Rosmarinic acid
Rosmarinic acid alleviates acetaminophen-induced hepatotoxicity by targeting Nrf2 signaling pathway
The MTT assay, wound healing assay, transwell migration assay, flow cytometry, and western blotting were employed to further evaluate RA’s protective effects on AILI and explore the mechanisms. The results indicated that RA pre-treatment lowered the serum ALT and AST levels, ameliorated the histological damage to the liver, and reduced ROS generation and the production of IL-1β and IL-18 in the liver tissues in APAP-treated mice. Moreover, pre-treatment with RA could promote the cell viability and migration ability and inhibit apoptosis in APAP-treated HepG2 cells. Mechanistically, RA could significantly suppress the APAP-induced activation of the NEK7-NLRP3 signaling pathway. Notably, depletion of Nrf2 by short hairpin RNA (shRNA) partly eliminated the protective effects of RA on AILI and the suppression of NEK7-NLRP3 signaling by RA. In summary, these results indicate that RA has a protective role against AILI through Nrf2-mediated inhibition of ROS production and suppression of the NEK7-NLRP3 pathway.
The results indicated that RA dramatically mitigated sickness behaviors and histologic brain damage in mice exposed to LPS. In addition, RA administration markedly promoted the expression of brain-derived neurotrophic factor (BDNF)/erythroid 2-related factor 2 (Nrf2), the key regulatory proteins for Nrf2 activation (p21 and p62), the downstream antioxidant enzymes (HO-1, NQO1, GCLC), the autophagy-related proteins (LC3II and Beclin1), and mitochondrial respiratory enzyme genes (ME1, IDH1, 6-PGDH), while reducing the expression of pro-inflammatory genes (CD44, iNOS, TNFα, IL-1β). Moreover, the double-label immunofluorescent analysis revealed that RA increased the fluorescence intensity of LC3 mostly co-localized with neurons and co-expressed with Nrf2. Taken together, our research found that RA could effectively alleviate sickness behaviors and nerve injury caused by neuroinflammation, and its protective effects were mediated by the Nrf2 signaling pathway, which reduced cellular oxidative stress, inflammation, mitochondrial respiratory function damage, and autophagy imbalance. Therefore, RA has the potential to prevent or treat sickness and depressive-like behaviors under conditions of neuroinflammation.
Salidroside
Treatment with salidroside dose-dependently attenuated cognitive impairment, reduced the accumulation of Aβ plaques and restored neuronal damage. Salidroside also suppressed the infiltration of CD8+T cells, oxidative stress, and inflammatory cytokines, and improved mitochondrial metabolism, iron metabolism, lipid metabolism, and redox in the SAMP8 mice brain. The administration of salidroside decreased iron deposition, reduced TFR1, and ACSL4 protein expression, upregulated SLC7A11, and GPX4 protein expression, and promoted the Nrf2/GPX4 axis activation.
In conclusion, neuronal ferroptosis and CD8+T cells are involved in the process of cognitive impairment in SAMP8 mice. Salidroside alleviates cognitive impairment and inhibits neuronal ferroptosis. The underlying mechanisms may involve the Nrf2/GPX4 axis activation and reduction in CD8+T cells infiltration. This study provides some evidence for the roles of salidroside in adaptive immunity and neuronal ferroptosis in SAMP8 mice.
Pulmonary fibrosis (PF) can severely disrupt lung function, leading to fatal consequences. Salidroside is a principal active ingredient of Rhodiola rosea and has recently been reported to protect against lung injures. The present study was aimed at exploring its therapeutic effects on PF. Lung fibrotic injuries were induced in SD rats by a single intratracheal instillation of 5 mg/kg bleomycin (BLM). Then, these rats were administrated with 50, 100, or 200 mg/kg salidroside for 28 days. BLM-triggered structure distortion, collagen overproduction, excessive inflammatory infiltration, and pro-inflammatory cytokine release, and oxidative stress damages in lung tissues were attenuated by salidroside in a dose-dependent manner.
Furthermore, salidroside was noted to inhibit IκBα phosphorylation and nuclear factor kappa B (NF-κB) p65 nuclear accumulation while activating Nrf2-antioxidant signaling in BLM-treated lungs. Downregulation of E-cadherin and upregulation of vimentin, fibronectin, and α-smooth muscle actin (α-SMA) indicated an epithelial-mesenchymal transition (EMT)-like shift in BLM-treated lungs. These changes were suppressed by salidroside. The expression of TGF-β1 and the phosphorylation of its downstream targets, Smad-2/-3, were enhanced by BLM, but weakened by salidroside. Additionally, salidroside was capable of reversing the recombinant TGF-β1-induced EMT-like changes in alveolar epithelial cells in vitro. Our study reveals that salidroside’s protective effects against fibrotic lung injuries are correlated to its anti-inflammatory, antioxidative, and antifibrotic properties.
Salidroside attenuates neuronal ferroptosis by activating the Nrf2/HO1 signaling pathway
Salidroside increased the cell viability and the level of MMP of Glu-injured HT22 cells, reduced the level of lipid peroxidation and ROS, and increased GPX4 and SLC7A11 protein expressions. These changes were not observed in siRNA Nrf2 transfected HT22 cells. Salidroside improved the ultrastructural changes in mitochondria of HT22 cells and Aβ1−42-induced AD mice, but not in Aβ1−42-induced Nrf2−/−AD mice. Salidroside increased protein expression levels of GPX4, HO1, and NQO1 and decreased protein expression of PTGS2 in Aβ1−42-induced AD mice but not in Aβ1−42-induced Nrf2−/−AD mice.
Salidroside plays a neuroprotective role by inhibiting neuronal ferroptosis in Aβ1−42-induced AD mice and Glu-injured HT22 cells, and its mechanism is related to activation of the Nrf2/HO1 signaling pathway.
Silibinin
Silibinin exhibits antidiabetic potential by preserving the mass and function of pancreatic β-cells through up-regulation of estrogen receptor-α (ERα) expression. However, the underlying protective mechanism of silibinin in pancreatic β-cells is still unclear. In the current study, we sought to determine whether ERα acts as the target of silibinin for the modulation of antioxidative response in pancreatic β-cells under high glucose and high fat conditions. Our in vivo study revealed that a 4-week oral administration of silibinin (100 mg/kg/day) decreased fasting blood glucose with a concurrent increase in levels of serum insulin in high-fat diet/streptozotocin-induced type 2 diabetic rats. Moreover, expression of ERα, NF-E2-related factor 2 (Nrf2), and heme oxygenase-1 (HO-1) in pancreatic β-cells in pancreatic islets was increased by silibinin treatment. Accordingly, silibinin (10 μM) elevated viability, insulin biosynthesis, and insulin secretion of high glucose/palmitate-treated INS-1 cells accompanied by increased expression of ERα, Nrf2, and HO-1 as well as decreased reactive oxygen species production in vitro. Treatment using an ERα antagonist (MPP) in INS-1 cells or silencing ERα expression in INS-1 and NIT-1 cells with siRNA abolished the protective effects of silibinin. Our study suggests that silibinin activates the Nrf2-antioxidative pathways in pancreatic β-cells through regulation of ERα expression.
Silibinin increases the expression of Nrf2, HO-1 and SOD2
Nrf2 and HO-1 (B) was examined by Western blot assay. (C) DCFH-DA probe was used to evaluate ROS production in INS-1 cells, and the fluorescence of oxidation product DCF was measured using a microplate reader with excitation at 485 nm and emission at 530 nm. (D, E) INS-1 cells were transfected with ERα siRNA or control siRNA, and then the expression of Nrf2, HO-1, and SOD2 as well as ROS production of cells with administration of silibinin and/or siRNA transfection were examined. Serum albumin (0.3% BSA) was present under all conditions. HG/PA, high glucose and palmitate/BSA; Sili, silibinin. Data are mean ± SEM of 3 independent experiments. *p<0.05 vs. control group; # p<0.05 vs. HG/PA group; & p<0.05 vs. HG/PA+Sili group.
Flavolignans from Silymarin as Nrf2 Bio activators and Their Therapeutic Applications
Silymarin (SM) is a mixture of flavolignans extracted from the seeds of species derived from Silybum marianum, commonly known as milk thistle or St. Mary’sthistle. These species have been widely used in the treatment of liver disorders in traditional medicine since ancient times. Several properties had been attributed to the major SM flavolignans components, identified as silybin, isosilybin, silychristin, isosilychristin, and silydianin. Previous research reported antioxidant and protective activities, which are probably related to the activation of the nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2), known as a master regulator of the cytoprotector response. Nrf2 is a redox-sensitive nuclear transcription factor able to induce the downstream-associated genes. The disruption of Nrf2 signaling has been associated with different pathological conditions. Some identified phytochemicals from SM had shown to participate in the Nrf2 signaling pathway; in particular, they have been suggested as activators that disrupt interactions in the Keap1-Nrf2 system, but also as antioxidants or with additional actions regarding Nrf2 regulation. Thus, the study of these molecules makes them appear attractive as novel targets for the treatment or prevention of several diseases.
Silymarin
Flavolignans from Silymarin as Nrf2 Bio activators and Their Therapeutic Applications
Silymarin (SM) is a mixture of flavolignans extracted from the seeds of species derived from Silybum marianum, commonly known as milk thistle or St. Mary’sthistle. These species have been widely used in the treatment of liver disorders in traditional medicine since ancient times. Several properties had been attributed to the major SM flavolignans components, identified as silybin, isosilybin, silychristin, isosilychristin, and silydianin. Previous research reported antioxidant and protective activities, which are probably related to the activation of the nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2), known as a master regulator of the cytoprotector response. Nrf2 is a redox-sensitive nuclear transcription factor able to induce the downstream-associated genes. The disruption of Nrf2 signaling has been associated with different pathological conditions. Some identified phytochemicals from SM had shown to participate in the Nrf2 signaling pathway; in particular, they have been suggested as activators that disrupt interactions in the Keap1-Nrf2 system, but also as antioxidants or with additional actions regarding Nrf2 regulation. Thus, the study of these molecules makes them appear attractive as novel targets for the treatment or prevention of several diseases.
Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases
Studies support the involvement of oxidative stress in the pathogenesis and development of CVDs like hypertension, atherosclerosis, ischemia, vascular dysfunction, Cardiotoxicity, cardiomyopathies and heart failure. Substantial clinical trials have been conducted to examine the protective effects of silymarin antioxidant in cardiovascular diseases. Silymarin shows a wide range of mechanisms in preventing the CVDs by increasing enzymatic antioxidants, mitochondrial enzymes and expression of Nrf2.
Soy isoflavone
Soy isoflavones (SIF) are soybean phytochemicals that are considered to be biologically active components that protect from neurodegenerative diseases. In this study, the therapeutic effect of SIF was evaluated in a diabetic Goto-Kakizaki (GK) rat model. Twenty male GK rats were randomly divided into diabetes mellitus (DM) model group and SIF+DM group (n=10 in each group). Twenty age-matched male Wistar rats were randomly divided into control group (CON group) and CON+SIF group, with 10 rats in each group. The learning and memory functions of the animals were determined by the Morris water maze (MWM) test. Hematoxylin-eosin staining (HE) was performed to examine pyramidal neuron loss in the CA1 area of the hippocampus. Markers of oxidative stress (OS) were measured to evaluate oxidative stress-mediated injury. RT-PCR and western blotting were used to analyze the expression of nuclear factorerythroid2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1) and NAD(P)H dehydrogenase quinone1 (NQO1). Treatment with SIF for 4 weeks alleviated the cognitive dysfunction of the GK rats as determined by the MWM test. Moreover, SIF treatment also reduced diabetes-related oxidative reactions. In addition, SIF enhanced the expression of Nrf2, HO-1 and NQO1, suggesting a potential antioxidation mechanism for the effect of SIF. These findings suggest that SIF can be considered candidates for inhibiting the progression of diabetes-induced cognitive dysfunction, provide novel insights into the antioxidant effect of SIF and further strengthen the link between oxidative stress and diabetes.
Sulforaphane
KEAP1 and done? Targeting the Nrf2 pathway with sulforaphane
Sulforaphane, as a pure chemical, protects against chemical-induced skin, oral, stomach, colon, lung and bladder carcinogenesis and in genetic models of colon and prostate carcinogenesis. In many of these settings the antitumorigenic efficacy of sulforaphane is dampened in Nrf2-disrupted animals. Broccoli preparations rich in glucoraphanin or sulforaphane exert demonstrable pharmacodynamic action in over a score of clinical trials. Measures of Nrf2 pathway response and function are serving as guideposts for the optimization of dose, schedule and formulation as clinical trials with broccoli-based preparations become more commonplace and more rigorous in design and implementation.
The recognition that food-derived nonnutrient molecules can modulate gene expression to influence intracellular molecular mechanisms has seen the emergence of the fields of nutrigenomics and nutrigenetics. The aim of this review is to describe the properties of nutrigenomic activators of transcription factor Nrf2, comparing the potential for sulforaphane and other phytochemicals to demonstrate clinical efficacy as complementary medicines. Broccoli-derived sulforaphane emerges as a phytochemical with this capability, with oral doses capable of favourably modifying genes associated with chemoprevention. Compared with widely used phytochemical-based supplements like curcumin, silymarin, and resveratrol, sulforaphane more potently activates Nrf2 to induce the expression of a battery of cytoprotective genes. By virtue of its lipophilic nature and low molecular weight, sulforaphane displays significantly higher bioavailability than the polyphenol-based dietary supplements that also activate Nrf2. Nrf2 activation induces cytoprotective genes such as those playing key roles in cellular defense mechanisms including redox status and detoxification. Both its high bioavailability and significant Nrf2 inducer capacity contribute to the therapeutic potential of sulforaphane-yielding supplements.
Sulforaphane and Other Nutrigenomic Nrf2 Activators
The aim of this review is to describe the properties of nutrigenomic activators of transcription factor Nrf2, comparing the potential for sulforaphane and other phytochemicals to demonstrate clinical efficacy as complementary medicines. Broccoli-derived sulforaphane emerges as a phytochemical with this capability, with oral doses capable of favourably modifying genes associated with chemoprevention. Compared with widely used phytochemical-based supplements like curcumin, silymarin, and resveratrol, sulforaphane more potently activates Nrf2 to induce the expression of a battery of cytoprotective genes.
By virtue of its lipophilic nature and low molecular weight, sulforaphane displays significantly higher bioavailability than the polyphenol-based dietary supplements that also activate Nrf2. Nrf2 activation induces cytoprotective genes such as those playing key roles in cellular defense mechanisms including redox status and detoxification. Both its high bioavailability and significant Nrf2 inducer capacity contribute to the therapeutic potential of sulforaphane-yielding supplements.
Tangeretin
The results of molecular docking suggested that tangeretin bound at the top of the central pore of Kelch-like ECH-associated protein 1 (Keap1) Kelch domain, and the hydrophobic and hydrogen bond interactions contributed to their stable binding. Herein, the regulation of Nrf2-ARE pathway by tangeretin was explored in the human embryonic kidney cell line HEK293T, which is relatively easy to be transfected. Upon binding to tangeretin, Nrf2 translocated to the nucleus of HEK293T cells, which in turn activated the Nrf2-ARE pathway. Luciferase reporter gene analysis showed that tangeretin significantly induced ARE-mediated transcriptional activation.
Real-time PCR and Western blot assays showed that tangeretin induced the gene and protein expressions of Nrf2-mediated targets, including heme oxygenase 1 (HO-1), nicotinamide adenine dinucleotide phosphate (NADPH) quinone dehydrogenase 1 (NQO1), and glutamate-cysteine ligase (GCLM). In addition, tangeretin could effectively scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals. In summary, tangeretin may be a potential antioxidant via activating the Nrf2-ARE pathway.
Taxifolin
Nuclear factor erythroid-2 related factor 2 (Nrf2) is a vital transcription factor that regulates the anti-oxidative defense system. Previous reports suggested that the expression of the Nrf2 gene can be regulated by epigenetic modifications. The potential epigenetic effect of taxifolin (TAX), a potent cancer chemopreventive agent, in skin cancer chemoprotection is unknown. In this study, we investigated how Nrf2 is epigenetically regulated by TAX in JB6 P+ cells. TAX was found to inhibit the 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced colony formation of JB6 P+ cells. TAX induced antioxidant response element (ARE)-luciferase activity in HepG2-C8 cells and up-regulated mRNA and protein levels of Nrf2 and its downstream genes heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase 1 (NQO1), in JB6 P+ cells. Furthermore, bisulfite genomic sequencing revealed that TAX treatment reduces the methylation level of the first 15 CpGs sites in the Nrf2 promoter. Western blotting showed that TAX inhibits the expression levels of DNA methyltransferase (DNMT) and histone deacetylase (HDAC) proteins. In summary, our results revealed that TAX can induce expression of Nrf2 and its downstream target genes in JB6 P+ cells by CpG demethylation. These finding suggest that TAX may exhibit a skin cancer preventive effect by activating Nrf2 via an epigenetic pathway.
Results: TAX suppresses IL-1β triggered secretion of inflammatory agents, chondrocyte apoptosis, and ECM degradation, contributing to the remodeling of the cartilage microenvironment. In vivo experiment results demonstrated that TAX counteracted cartilage degeneration induced by DMM in rats. Mechanistic investigations revealed that TAX hinders OA development by reducing NF-κB activation and ROS production through the activation of the Nrf2/HO-1 axis.
Conclusion: TAX reshapes the articular cartilage microenvironment by suppressing inflammation, mitigating apoptosis, and decreasing ECM degradation through the activation of the Nrf2 pathway. As a result, pharmacological activation of the Nrf2 pathway by TAX holds potential clinical significance in remodeling the joint microenvironment for OA treatment.
tert-Butylhydroquinone
The nuclear factor erythroid 2-related factor 2 (Nrf2) is required to combat increases in oxidative stress. The chemical compound tert-butylhydroquinone (tBHQ) can downregulate Kelch-like ECH-associated protein 1 (Keap1), a repressor of Nrf2, thus maintaining the stability of Nrf2. tBHQ can also increase intracellular “free” zinc in human bronchial epithelial (16HBE) cells. We aim to investigate whether the intracellular free zinc change plays a role in Nrf2 activation. tBHQ exposure dose-dependently increases intracellular free zinc concentrations within 30 min in 16HBE cells by mobilizing intracellular zinc pools. Active Nrf2 and the antioxidant enzyme heme oxygenase-1 (HO-1) increase at 3 h after tBHQ treatment. Chelating intracellular free zinc with tetrakis-(2-pyridylmethyl)ethylenediamine (TPEN) during tBHQ exposure partially abrogates the tBHQ-induced activation of Nrf2 and HO-1 expression, while Keap1 is further decreased. These results indicate that tBHQ-induced stability of Nrf2 is associated with the intracellular free zinc level.
Tert-butylhydroquinone induces mitochondrial oxidative stress causing Nrf2 activation
Tert-butylhydroquinone (tBHQ), the major metabolite of butylated hydroxyanisole, induces an antioxidant response through the redox-sensitive transcription factor, nuclear factor-E2-related factor-2 (Nrf2). However, the mechanism by which tBHQ induces Nrf2 activity is not entirely understood. Here, we show that tBHQ preferentially alters the redox status in the mitochondrial compartment in HeLa cells. HeLa cells treated with tBHQ showed a preferential oxidation of mitochondrial thioredoxin-2 (Trx2), while cellular glutathione and cytosolic thioredoxin-1 were not affected. Preferential mitochondrial oxidation by tBHQ was supported by detection of reactive oxygen species (ROS) specific to this compartment. To determine the role of Trx2 in regulating downstream effects of tBHQ, HeLa cells were transiently transfected with an empty, Trx2, or C93S (Cys93Ser) Trx2 dominant-negative mutant expression vector. Overexpression of Trx2 decreased basal mitochondrial ROS production, whereas expression of C93S Trx2 enhanced it. In addition, under untreated conditions, expression of C93S Trx2 led to an increase in the basal activities of Nrf2. With tBHQ treatments, Trx2 overexpression suppressed Nrf2 accumulation and activity, whereas expression of C93S Trx2 had no effect on the degree of inducibility or Nrf2 accumulation but did increase the overall activity of Nrf2. Quantitative polymerase chain reaction analysis of Nrf2-regulated gene expression corroborate Trx2 control of tBHQ-mediated Nrf2 activation. These data show a compartment-specific effect where tBHQ-induced Nrf2 signaling is mediated by Trx2 and suggest that antioxidant status in various compartments would provide different levels of control of redox signaling.
Turnips extract
Antioxidant and Anti-Inflammatory Activities of High-Glucosinolate-Synthesis Lines of Brassica rapa
Excessive oxidative stress and inflammatory responses are associated with the development of various diseases, including cancer. Glucosinolates (GSLs) are phytochemicals known for their antioxidant properties, and doubled haploid lines (DHLs) of Brassica rapa with high GSL contents (HGSL) were intentionally developed from two edible subspecies of Brassica rapa: B. rapa subsp. trilocularis and B. rapa subsp. chinensis. The purpose of the present study is to assess the capacity of HGSL DHLs to mitigate oxidative stress and inflammation in lipopolysaccharide (LPS)-stimulated RAW264.7 cells, compared to pak choi as a parental control. Our findings demonstrate that HGSL DH lines effectively suppressed the expression of inducible nitric oxide synthase, leading to the reduced levels of nitric oxide at non-toxic concentrations. Additionally, these lines exhibited scavenging activity against reactive oxygen species and free radicals. The enhanced antioxidant capacity of HGSL DHLs was mechanistically attributed to the upregulation of antioxidant enzymes, such as NADPH quinone oxidoreductase 1 (NQO1), the glutamate–cysteine ligase catalytic subunit (GCLC), and heme oxygenase-1 (HMOX1). Furthermore, we confirmed that these effects were mediated through the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway via p38 phosphorylation. Moreover, HGSL DHLs demonstrated inhibitory effects on pro-inflammatory cytokines and signal transducers and activators of transcription 3 (STAT3) phosphorylation. Collectively, our results indicate that HGSL DHLs possess better antioxidant and anti-inflammatory properties compared to the parental control pak choi in LPS-stimulated RAW264.7 cells, suggesting that HGSL DHLs of Brassica rapa could be considered as a beneficial daily vegetable for reducing the risk of inflammation-associated diseases.
Brain cell damage that results from oxidative toxicity contributes to neuronal degeneration. The transcription factor nuclear factor‑E2‑related factor 2 (Nrf2) regulates the expression of heme oxygenase (HO)‑1 and glutathione (GSH), and serves a key role in the pathogenesis of neurological diseases. Brassica rapa is a turnip that is unique to Ganghwa County, and is used mainly for making kimchi, a traditional Korean food. In the current study, brassicaphenanthrene A (BrPA) from B. rapa was demonstrated to exhibit protective effects against neurotoxicity induced by glutamate via Nrf2‑mediated HO‑1 expression. Similarly, BrPA increased the expression of cellular glutathione and glutamine‑cysteine ligase genes. Furthermore, BrPA caused the nuclear translocation of Nrf2 and increased antioxidant response element (ARE) promoter activity. Nrf2 also mediated HO‑1 induction by BrPA through the PI3K/Akt and JNK regulatory pathways. The results of the present study indicated the neuroprotective effect of BrPA, a natural food component from B. rapa.
Ursolic Acid
Results: In EAM mice, UA intervention significantly reduced the degree of inflammatory infiltration and myocardial fibrosis while improving cardiac function. Mechanistically, UA reduced myocardial injury by inhibiting oxidative stress (as demonstrated by a decrease of superoxide and normalization of pro- and antioxidant enzyme levels). Interestingly, UA intervention upregulated the expression of antioxidant factors such as Nrf2 and HO-1. In vitro experiments, specific Nrf2 inhibitors reversed the antioxidant and antiapoptotic effects of ursolic acid, which further suggested that the amelioration of EAM by UA was in a Nrf2/HO-1 pathway-dependent manner.
Conclusion: These findings indicate that UA is a cardioprotective traditional Chinese medicine formula that reduces EAM-induced cardiac injury by up-regulating Nrf2/HO-1 expression and suppressing oxidative stress, making it a promising therapeutic strategy for the treatment of EAM.
Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice
Results: UA treatment significantly improved neurological deficit and reduced infarct size in WT mice after MCAO. Administration of UA also decreased the product of lipid peroxidation, promoted the activation of Nrf2 pathway and decreased the expression of TLR4 and NF-KB after stroke in WT mice. However, Nrf2(-/-) mice demonstrated more severe neurologic deficits, infarct size and inflammatory damage after MCAO, and did not benefit from the protective effect of UA.
Conclusion: The results indicated that UA protected the brain against ischemic injury in mice by anti-oxidative and anti-inflammatory effects after MCAO. activation of the Nrf2 pathway contributes to the neuroprotective effects induced by UA in cerebral ischemia.
Ursolic acid (UA), a well-known natural triterpenoid found in abundance in blueberries, cranberries and apple peels, has been reported to possess many beneficial health effects. These effects include anti-cancer activity in various cancers, such as skin cancer. Skin cancer is the most common cancer in the world. Nuclear factor E2-related factor 2 (Nrf2) is a master regulator of anti- oxidative stress response with anti-carcinogenic activity against UV- and chemical-induced tumor formation in the skin. Recent studies show that epigenetic modifications of Nrf2 play an important role in cancer prevention.
However the epigenetic impact of UA on Nrf2 signaling remains poorly understood in skin cancer. In this study, we investigated the epigenetic effects of UA on mouse epidermal JB6 P+ cells. UA inhibited cellular transformation by 12-O-tetradecanoylphorbol-13-acetate (TPA) at a concentration at which the cytotoxicity was no more than 25%. Under this condition, UA induced the expression of the Nrf2-mediated detoxifying/antioxidant enzymes heme oxygenase-1 (HO-1), NAD(P)H:quinone oxidoreductase 1 (NQO1), and UDP-glucuronosyltransferase 1A1 (UGT1A1). DNA methylation analysis revealed that UA demethylated the first 15 CpG sites of the Nrf2 promoter region, which correlated with the re-expression of Nrf2. Furthermore, UA reduced the expression of epigenetic modifying enzymes, including the DNA methyltransferases (DNMTs) DNMT1 and DNMT3a and the histone deacetylases (HDACs) HDAC1, 2, 3, and 8 (Class I) and HDAC6 and 7 (Class II), and HDAC activity. Taken together, these results suggest that the epigenetic effects of the triterpenoid UA could potentially contribute to its beneficial effects, including the prevention of skin cancer.
Vitamin D
Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats
Results: Although maxacalcitol reduced albuminuria and mesangial matrix expansion, no significant differences were observed in blood pressure and creatinine clearance among the 3 treatment groups. Systemic and intrarenal oxidative stress was reduced by maxacalcitol therapy. Expressions of nuclear factor-κB and nicotinamide adenine dinucleotide phosphate oxidase in the kidney also decreased in the insulin-treated and maxacalcitol-treated groups but increased in the vehicle-alone group. In addition, the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) decreased and Kelch-like erythroid cell-derived protein with CNC homology (ECH)-associated protein 1 (Keap1) increased in the vehicle-treated group; however, these expressions were restored in the maxacalcitol- and insulin-treated groups.
Conclusions: It is suggested that maxacalcitol attenuates the progression of diabetic nephropathy by suppression of oxidative stress and amelioration of the Nrf2-Keap1 pathway in nonobese type 2 diabetes without significant changes in blood pressure and glomerular filtration rate.
Hyperglycemia contributes to the development of cognition impairment and related disorders, induces oxidative stress in neuronal cells; thereby, impairs normal signaling mechanisms involved in cognition processes. Studies have shown a significant decrease in the vitamin D in individuals with hyperglycemia and cognition impairment. But whether supplementing vitamin D has any beneficiary impact on mitigating hyperglycemia-induced cognition impairment is unknown. We have first tested the impact of hyperglycemia on the induction of cognition deficiency in a zebrafish model. Next, the molecular mechanisms related to oxidative stress, which are deregulated in hyperglycemic zebrafish brains, have been explored. Subsequently, the impact of supplementing the water with vitamin D and a known activator of nuclear factor erythroid-2 related factor 2 (Nrf2) i.e., sulforaphane (SFN) on learning and memory functions were assessed. We showed a significant increase in the oxidative stress in the brain tissue of zebrafish residing in hyperglycemic water (111 mM glucose). Addition of vitamin D and SFN increased Nrf2, but differentially modulated its target genes (NQO1, SOD, GPx etc) activity in zebrafish and neuronal cell lines thereby improved the hyperglycemia-induced decline of cognition impairment. Mechanistically, vitamin D binds to the Keap1 protein; thereby, interfering with its binding to Nrf2, which leads to the activation of antioxidant mechanisms in the cells. In summary, reducing the oxidative stress through vitamin D treatment is a possible option for controlling the cognition impairment in diabetic population, but studies testing this possibility in clinical trials are currently needed.
Withania Somnifera
Bioactive phytochemicals in Rosmarinus officinalis, Withania somnifera, and Sophora japonica have a long history of human use to promote health. In this study we examined the cellular effects of a combination of extracts from these plant sources based on specified levels of their carnosol/carnosic acid, withaferin A, and luteolin levels, respectively. Individually, these bioactive compounds have previously been shown to activate the nuclear factor erythroid 2-related factor 2 (Nrf2) transcription factor, which binds to the antioxidant response element (ARE) and regulates the expression of a wide variety of cytoprotective genes. We found that combinations of these three plant extracts act synergistically to activate the Nrf2 pathway, and we identified an optimized combination of the three agents which we named PB125 for use as a dietary supplement. Using microarray, quantitative reverse transcription-PCR, and RNA-seq technologies, we examined the gene expression induced by PB125 in HepG2 (hepatocellular carcinoma) cells, including canonical Nrf2-regulated genes, noncanonical Nrf2-regulated genes, and genes which appear to be regulated by non-Nrf2 mechanisms. Ingenuity Pathway Analysis identified Nrf2 as the primary pathway for gene expression changes by PB125. Pretreatment with PB125 protected cultured HepG2 cells against an oxidative stress challenge caused by cumene hydroperoxide exposure, by both cell viability and cell injury measurements. In summary, PB125 is a phytochemical dietary supplement comprised of extracts of three ingredients, Rosmarinus officinalis, Withania somnifera, and Sophora japonica, with specified levels of carnosol/carnosic acid, withaferin A, and luteolin, respectively. Each ingredient contributes to the activation of the Nrf2 pathway in unique ways, which leads to upregulation of cytoprotective genes and protection of cells against oxidative stress and supports the use of PB125 as a dietary supplement to promote healthy aging.
Increased production of reactive oxygen species has been implicated in the pathogenesis of cardiovascular disease (CVD), and enhanced endogenous antioxidants have been proposed as a mechanism for regulating redox balance. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is a transcriptional regulator of phase II antioxidant enzymes, and activation of Nrf2 has been suggested to be an important step in attenuating oxidative stress associated with CVD. A well-defined combination of five widely studied medicinal plants derived from botanical sources (Bacopa monniera, Silybum marianum (milk thistle), Withania somnifera (Ashwagandha), Camellia sinensis (green tea), and Curcuma longa (turmeric)) has been shown to activate Nrf2 and induce phase II enzymes through the antioxidant response element. The purpose of these experiments was to determine if treatment of cardiomyocytes with this phytochemical composition, marketed as Protandim, activates Nrf2, induces phase II detoxification enzymes, and protects cardiomyocytes from oxidant-induced apoptosis in a Nrf2-dependent manner. In cultured HL-1 cardiomyocytes, phytochemical treatment was associated with nuclear accumulation of Nrf2, significant induction of phase II enzymes, and concomitant protection against hydrogen peroxide-induced apoptosis. The protection against oxidant stress was abolished when Nrf2 was silenced by shRNA, suggesting that our phytochemical treatment worked through the Nrf2 pathway. Interestingly, phytochemical treatment was found to be a more robust activator of Nrf2 than oxidant treatment, supporting the use of the phytochemicals as a potential treatment to increase antioxidant defenses and protect heart cells against an oxidative challenge.
Withaferin A is also a potent inducer of nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor that regulates the expression of antioxidant enzyme genes involved in the response to oxidative stress through activation of the PTEN/PI3K/Akt pathway, exerting a cytoprotective role [68, 69]. Nrf2 activation creates downstream production of antioxidant enzymes such as catalase and SOD, glutathione (GSH) synthase, GSH peroxidase, GSH reductase, thioredoxin (Trx) and Trx reductase, and antioxidant proteins such as heat shock protein 70 (HSP70) and Heme oxygenase-1 (HO-1) [70]. Unlike vitamins and coenzymes, these enzymatic and protein antioxidants are not consumed in the redox reaction, providing a longer duration of action because they no longer required continuous production [71]. The antioxidant properties of the bioactive compounds of Ws in healthy individuals support its use to improve oxidative balance status and may prevent oxidative stress-associated diseases.
Wogonin
Wogonin alleviates liver injury in sepsis through Nrf2‐mediated NF‐κB signalling suppression
Sepsis is a life‐threatening organ dysfunction syndrome, and liver is a susceptible target organ in sepsis, because the activation of inflammatory pathways contributes to septic liver injury. oxidative stress has been documented to participate in septic liver injury, because it not only directly induces oxidative genotoxicity, but also exacerbates inflammatory pathways to potentiate damage of liver. Therefore, to ameliorate oxidative stress is promising for protecting liver in sepsis. Wogonin is the compound extracted from the medicinal plant Scutellaria baicalensis Geogi and was found to exert therapeutic effects in multiple inflammatory diseases via alleviation of oxidative stress. However, whether wogonin is able to mitigate septic liver injury remains unknown. Herein, we firstly proved that wogonin treatment could improve survival of mice with lipopolysaccharide (LPS)‐ or caecal ligation and puncture (CLP)‐induced sepsis, together with restoration of reduced body temperature and respiratory rate, and suppression of several pro‐inflammatory cytokines in circulation. Then, we found that wogonin effectively alleviated liver injury via potentiation of the anti‐oxidative capacity. To be specific, wogonin activated Nrf2 thereby promoting expressions of anti‐oxidative enzymes including NQO‐1, GST, HO‐1, SOD1 and SOD2 in hepatocytes. Moreover, wogonin‐induced Nrf2 activation could suppress NF‐κB‐regulated up‐regulation of pro‐inflammatory cytokines. Ultimately, we provided in vivo evidence that wogonin activated Nrf2 signalling, potentiated anti‐oxidative enzymes and inhibited NF‐κB‐regulated pro‐inflammatory signalling. Taken together, this study demonstrates that wogonin can be the potential therapeutic agent for alleviating liver injury in sepsis by simultaneously ameliorating oxidative stress and inflammatory response through the activation of Nrf2.
Wogonin exhibits potent chondroprotective potential by switching the signaling axis of matrix degradation from catabolic towards anabolic ends and inhibited the expression, production and activities of matrix degrading proteases including MMP-13, MMP-3, MMP-9, and ADAMTS-4 in OA chondrocytes, and blocked the release of s-GAG and COL2A1 in IL-1β-stimulated OA cartilage explants. Wogonin also elevated the expression of cartilage anabolic factors COL2A1 and ACAN in chondrocytes and inhibited the IL-1β-mediated depletion of COL2A1 and proteoglycan content in the matrix of cartilage explants. The suppressive effect of Wogonin was not mediated through the inhibition of MAPKs or NF-κB activation. Instead, Wogonin induced mild oxidative stress through the generation of ROS and depletion of cellular GSH, thereby modulating the cellular redox leading to the induction of Nrf2/ARE pathways through activation of ROS/ERK/Nrf2/HO-1-SOD2-NQO1-GCLC signaling axis in OA chondrocytes. Molecular docking studies revealed that Wogonin can disrupt KEAP-1/Nrf-2 interaction by directly blocking the binding site of Nrf-2 in the KEAP-1 protein. Genetic ablation of Nrf2 using specific siRNA, significantly abrogated the anti-inflammatory and chondroprotective potential of Wogonin in IL-1β-stimulated OA chondrocytes. Our data indicates that Wogonin exerts chondroprotective effects through the suppression of molecular events involved in oxidative stress, inflammation and matrix degradation in OA chondrocytes and cartilage explants. The study provides novel insights into the development of Nrf2 as a promising candidate and Wogonin as a therapeutic agent for the management of OA.
Wogonoside
Compared with those in NAFLD group, the liver mass, liver index and the LDL, TG, TC, IL-2, IL-6, TNF-α, MDA and NF-κB p65 levels were decreased, and the SOD and GSH-Px activities, and HDL, IκBα, Nrf2 and HO-1 contents were increased in wogonoside groups. Compared with those in the NAFLD group, wogonoside (5, 10 and 20 mg/kg) reduced AST (132.21 ± 14.62, 115.70 ± 11.32 and 77.94 ± 8.86 vs. 202.35 ± 19.58 U/L) and ALT (104.37 ± 11.92, 97.53 ± 10.12 and 56.74 ± 6.33 vs. 154.66 ± 14.23 U/L) activities in the serum.
Wogonoside has a protective effect against NAFLD in mice, which may be related to its anti-inflammation and inhibition of oxidative stress, suggesting that wogonoside may be a potential therapeutic agent for the treatment of NAFLD.
Results: In I/R group, mice had severe myocardial injury, however, pretreatment of wogonoside at doses of 20 and 40 mg/kg ameliorated the cardiac function, as evidenced by improving hemodynamic parameters. Besides, wogonoside could relieved the abnormality of cardiomyocytes structure, inflammatory reaction, apoptosis, and myocardial fibrosis. Importantly, wogonoside activated the Nrf2/HO-1 pathway, as demonstrated by increasing Nrf2 expression in nucleus and its downstream genes including HO-1 and NADPH quinone oxidoreductase-1 (NQO1). However, effects of wogonoside on cardioprotection were abolished by sh-Nrf2.
Conclusions: Wogonoside exerted the protective role against I/R-induced myocardial injury by suppression of apoptosis, inflammation, and fibrosis via activating Nrf2/HO-1 pathway.
Xanthohumol
The nuclear factor erythroid 2-related factor 2 (Nrf2), a master transcription factor controlling a series of cytoprotective genes, is closely associated with scavenging the reactive oxygen species and maintaining the intracellular redox balance. Accumulating evidence has indicated that activation of Nrf2 is efficient to block or retard oxidative stress mediated neurodegenerative disorders. Small molecules that contribute directly or indirectly to the Nrf2 activation thus are promising therapeutic agents. Herein, we screened xanthohumol and its analogues, and two analogues (11 and 12) were disclosed to possess low cytotoxicity and rescue PC12 cells from the hydrogen peroxide or 6-hydroxydopamine induced injuries.
Molecular mechanism studies demonstrated that compounds 11 and 12 are potent Nrf2 activators by promoting the nuclear accumulation of Nrf2 and enhancing the cellular antioxidant defense system. More importantly, genetically silencing the Nrf2 expression shuts down the observed cytoprotection conferred by both compounds, supporting the critical involvement of Nrf2 for the cellular actions of compounds 11 and 12.
Zeaxanthin
The protection of structure and function of RPE raised questions about the mechanism of protection. Zeaxanthin effectively protected against tert-butyl hydroperoxide-induced mitochondrial dysfunction and apoptosis in RPE cells through NF-E2-related factor 2 (Nrf2) activation [27]. Zeaxanthin was further reported to protect a rat model of oxidative damage by inducing Nrf2 and HO-1 [47]. In Sod2flox/floxVMD2-cre mice we expected greater oxidative stress in RPE since an essential antioxidant enzyme was deleted in the RPE. Our observation that zeaxanthin increased RPE expression of Nrf2 -regulated enzymes, including catalase, NAD(P)H quinone dehydrogenase 1and heme oxygenase 1 confirmed the induction of antioxidant enzyme mechanism in vivo. The mu class of glutathione S-transferase (GSTM1) enzyme functions in the detoxification of electrophilic compounds, and products of oxidative stress, by conjugation with glutathione [48]. Dietary supplementation of zeaxanthin also increased GSTM1 expression in Sod2flox/floxVMD2-cre mice. Since the ARE genes are regulated at the stage of transcription, we presume that the protein levels also increased, but we did not confirm this using immunological methods. Zou et al. also showed that oral supplementation of zeaxanthin induces Nrf2-mediated phase II enzymes in vitro and in vivo [27]. Not having the protein data to support increased antioxidant defense by RPE is a weakness in the current study and it will be explored in future studies.
Zeaxanthin induces Nrf2-mediated phase II enzymes in protection of cell death
Zeaxanthin (Zea) is a major carotenoid pigment contained in human retina, and its daily supplementation associated with lower risk of age-related macular degeneration. Despite known property of Zea as an antioxidant, its underlying molecular mechanisms of action remain poorly understood. In this study, we aim to study the regulation mechanism of Zea on phase II detoxification enzymes. In normal human retinal pigment epithelium cells, Zea promoted the nuclear translocation of NF-E2-related factor 2 (Nrf2) and induced mRNA and protein expression of phase II enzymes, the induction was suppressed by specific knockdown of Nrf2. Zea also effectively protected against tert-butyl hydroperoxide-induced mitochondrial dysfunction and apoptosis. Glutathione (GSH) as the most important antioxidant was also induced by Zea through Nrf2 activation in a time- and dose-dependent manner, whereas the protective effects of Zea were decimated by inhibition of GSH synthesis. Finally, Zea activated the PI3K/Akt and MAPK/ERK pathway, whereas only PI3K/Akt activation correlated with phase II enzymes induction and Zea protection. In further in vivo analyses, Zea showed effects of inducing phase II enzymes and increased GSH content, which contributed to the reduced lipid and protein peroxidation in the retina as well as the liver, heart, and serum of the Sprague–Dawley rats. For the first time, Zea is presented as a phase II enzymes inducer instead of being an antioxidant.
By activating Nrf2-mediated phase II enzymes, Zea could enhance anti-oxidative capacity and prevent cell death both in vivo and in vitro.
Zerumbone
ZER treatment prior to low dose of UVA exposure increased cell viability. UVA-induced ROS generation was remarkably suppressed by ZER with parallel inhibition of MMP-1 activation and collagen III degradation. This was due to the inhibition of AP-1 (c-Fos and c-Jun) translocation. Furthermore, ZER alleviated UVA-induced SA-β-galactosidase activity. Dose- or time-dependent increase of antioxidant genes, HO-1 and γ-GCLC by ZER, was associated with increased expression and nuclear accumulation of Nrf2 as well as decreased cytosolic Keap-1 expressions. Altered luciferase activity of ARE could explain the significance of Nrf2/ARE pathway underlying the dermatoprotective properties of ZER. Pharmacological inhibition of various signaling pathways suppressed nuclear Nrf2 activation in HSF cells confirming that Nrf2 translocation was mediated by ERK, JNK, PI3K/AKT, PKC, AMPK, casein kinase II, and ROS signaling pathways.
Moreover, increased basal ROS levels and Nrf2 translocation seem crucial in ZER-mediated Nrf2/ARE signaling pathway. This was also evidenced from Nrf2 knocked-out studies in which ZER was not able to suppress the UVA-induced ROS generation in the absence of Nrf2. This study concluded that in the treatment of UVA-induced premature skin aging, ZER may consider as a desirable food supplement for skin protection and/or preparation of skin care products.
β-Carotene
Acute spinal cord injury (SCI) results in long-lasting functional impairments through both mechanical damage as well as secondary mechanisms, with limited available therapeutic options. β-Carotene has been demonstrated to exert biological and pharmacological activities. We aimed to examine the protective effects of β-carotene in a SCI rat model. We tested the hind-limb locomotor function, neuro-inflammation, oxidative stress, astrocyte activation and nuclear factor–κB (NF-κB) pathway activation of SCI rats, with or without β-carotene treatment. β-Carotene substantially improved locomotion that was reduced by SCI. β-Carotene also relieved SCI-induced oxidative stress via regulation of reactive oxygen species, malondialdehyde, nitric oxide, and superoxide dismutase, as well as restored SCI-suppressed protein expressions of Nrf2 and HO-1. Additionally, β-carotene decreased the generation of pro-inflammatory cytokines, including tumor necrosis factor-α, interleukin-1β, interleukin-18 and cyclooxygenase-2, and inhibited the activation of astrocyte in the spinal cord. Furthermore, β-carotene treatment markedly inhibited the NF-κB pathway activation. Our findings demonstrated that β-carotene effectively reduced the progression of secondary injury events following SCI through preventing NF-κB pathway activation. Therefore, β-carotene may be an effective candidate for treating SCI.
Skin ageing is influenced by several factors including environmental exposure and hormonal changes. Reactive oxygen species (ROS), which mediate many of the effects of these factors, induce inflammatory processes in the skin and increase the production of matrix metalloproteinases (MMPs) in dermal fibroblasts, which leads to collagen degradation. Several studies have shown the protective role of estrogens and a diet rich in fruits and vegetables on skin physiology. Previous studies have shown that dietary carotenoids and polyphenols activate the cell’s antioxidant defense system by increasing antioxidant response element/Nrf2 (ARE/Nrf2) transcriptional activity and reducing the inflammatory response. The aim of the current study was to examine the protective effect of such dietary-derived compounds and estradiol on dermal fibroblasts under oxidative stress induced by H2O2. Human dermal fibroblasts were used to study the effect of H2O2 on cell number and apoptosis, MMP-1, and pro-collagen secretion as markers of skin damage. Treatment of cells with H2O2 led to cell death, increased secretion of MMP-1, and decreased pro-collagen secretion. Pre-treatment with tomato and rosemary extracts, and with estradiol, reversed the effects of the oxidative stress. This was associated with a reduction in intracellular ROS levels, probably through the measured increased activity of ARE/Nrf2. Conclusions: This study indicates that carotenoids, polyphenols, and estradiol protect dermal fibroblasts from oxidative stress-induced damage through a reduction in ROS levels.
Carotenoids activate the antioxidant response element transcription system
Epidemiologic studies have found an inverse association between consumption of tomato products and the risk of certain types of cancers. However, the mechanisms underlying this relationship are not completely understood. One mechanism that has been suggested is induction of phase II detoxification enzymes. Expression of phase II enzymes is regulated by the antioxidant response element (ARE) and the transcription factor Nrf2 (nuclear factor E2-related factor 2). In this study, we determined the role of this transcription system in the induction of phase II enzymes by carotenoids. We found that in transiently transfected cancer cells, lycopene transactivated the expression of reporter genes fused with ARE sequences. Other carotenoids such as phytoene, phytofluene, beta-carotene, and astaxanthin had a much smaller effect. An increase in protein as well as mRNA levels of the phase II enzymes NAD(P)H:quinone oxidoreductase and gamma-glutamylcysteine synthetase was observed in nontransfected cells after carotenoid treatment. Ethanolic extract of lycopene containing unidentified hydrophilic derivatives of the carotenoid activated ARE with similar potency to lycopene. The potency of the carotenoids in ARE activation did not correlate with their effect on intracellular reactive oxygen species and reduced glutathione level, which may indicate that ARE activation is not solely related to their antioxidant activity. Nrf2, which is found predominantly in the cytoplasm of control cells, translocated to the nucleus after carotenoid treatment. Interestingly, part of the translocated Nrf2 colocalized with the promyelocytic leukemia protein in the promyelocytic leukemia nuclear bodies. The increase in phase II enzymes was abolished by a dominant-negative Nrf2, suggesting that carotenoid induction of these proteins depends on a functional Nrf2 and the ARE transcription system.
Mandatory FDA Disclaimer: As with any supplement, consult your medical practitioner prior to using this product if you are pregnant, may become pregnant, breastfeeding, taking medication, other dietary supplements or have a medical condition. These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. *Daily Value has not been established.
| Size | 25g, 100g |
|---|
3 reviews for NRF2 ACTIVATOR (Antioxidant Defense) – New!
Related products
-
INTERSTELLAR SPICE (Polyphenol Powerhouse)
Rated 5.00 out of 55.0 $75.00 – $275.00Price range: $75.00 through $275.00



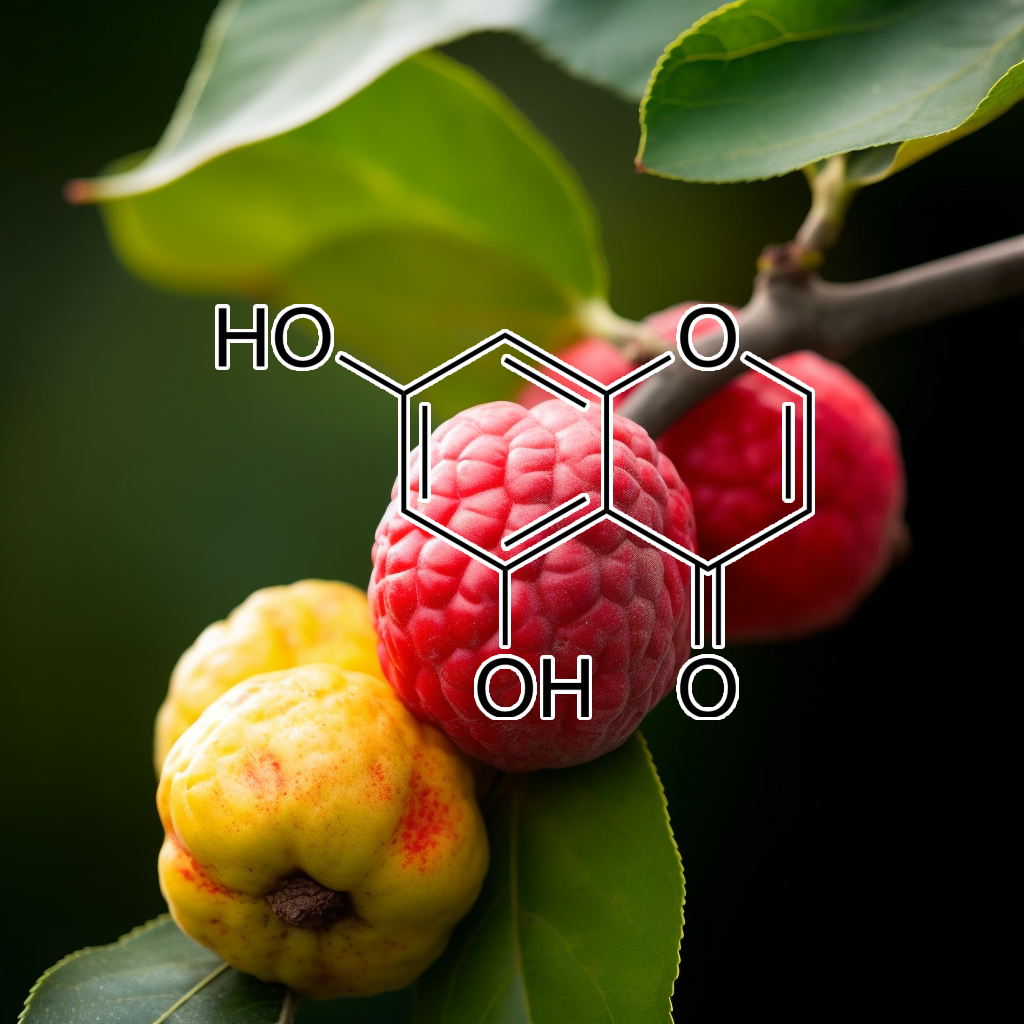
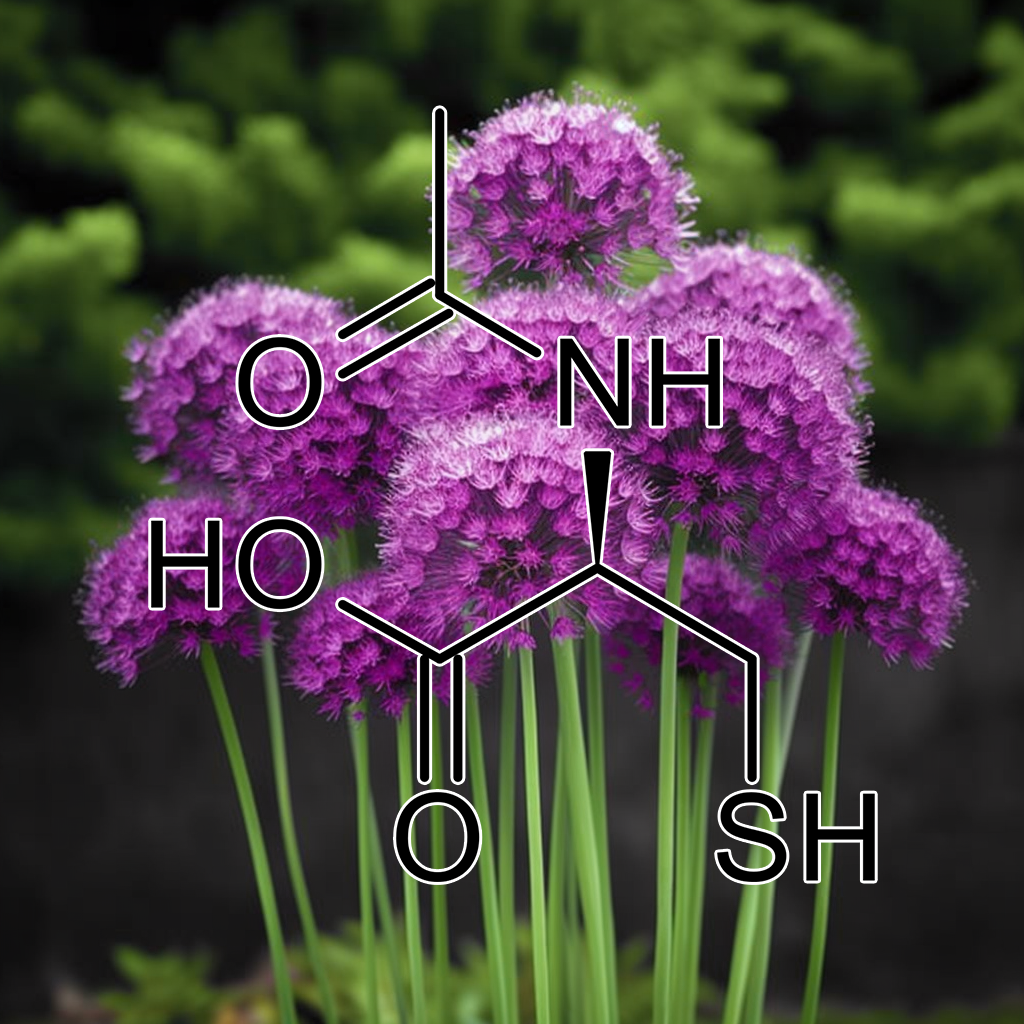
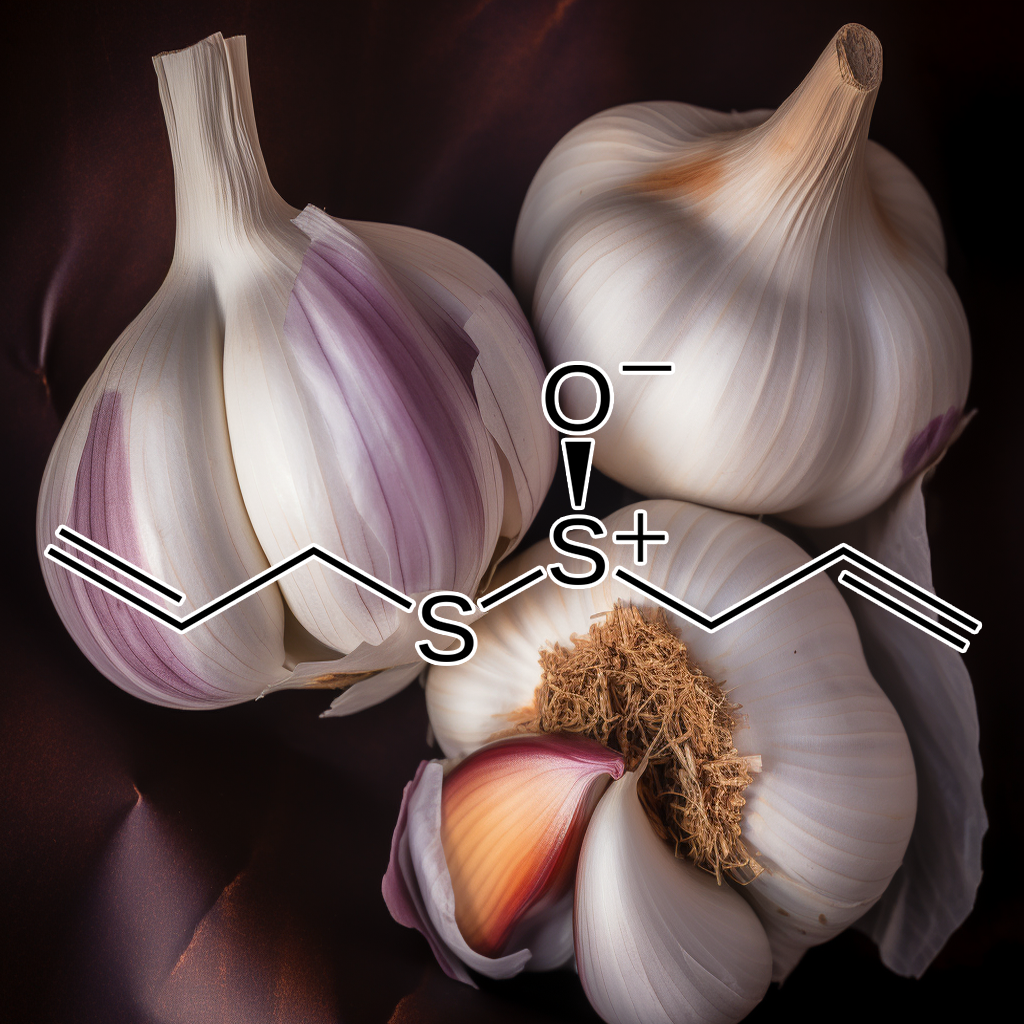

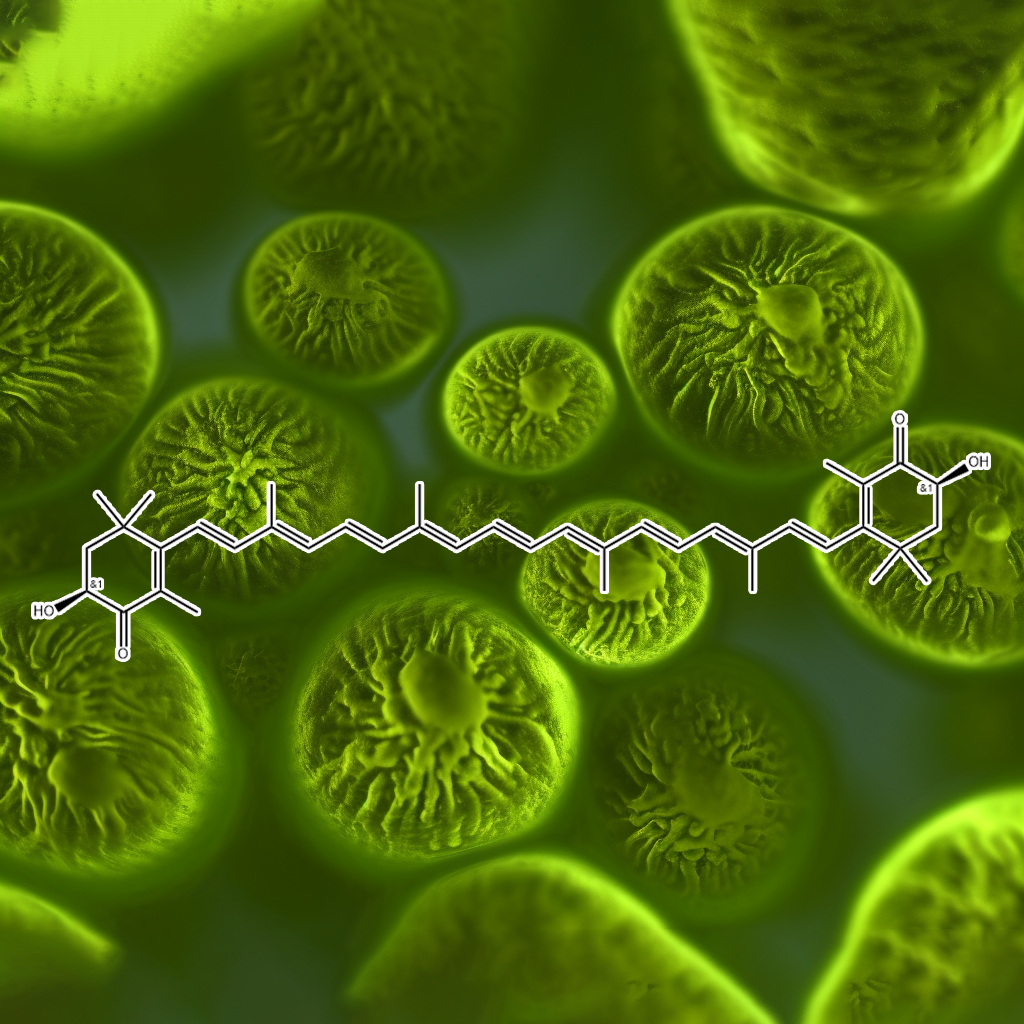





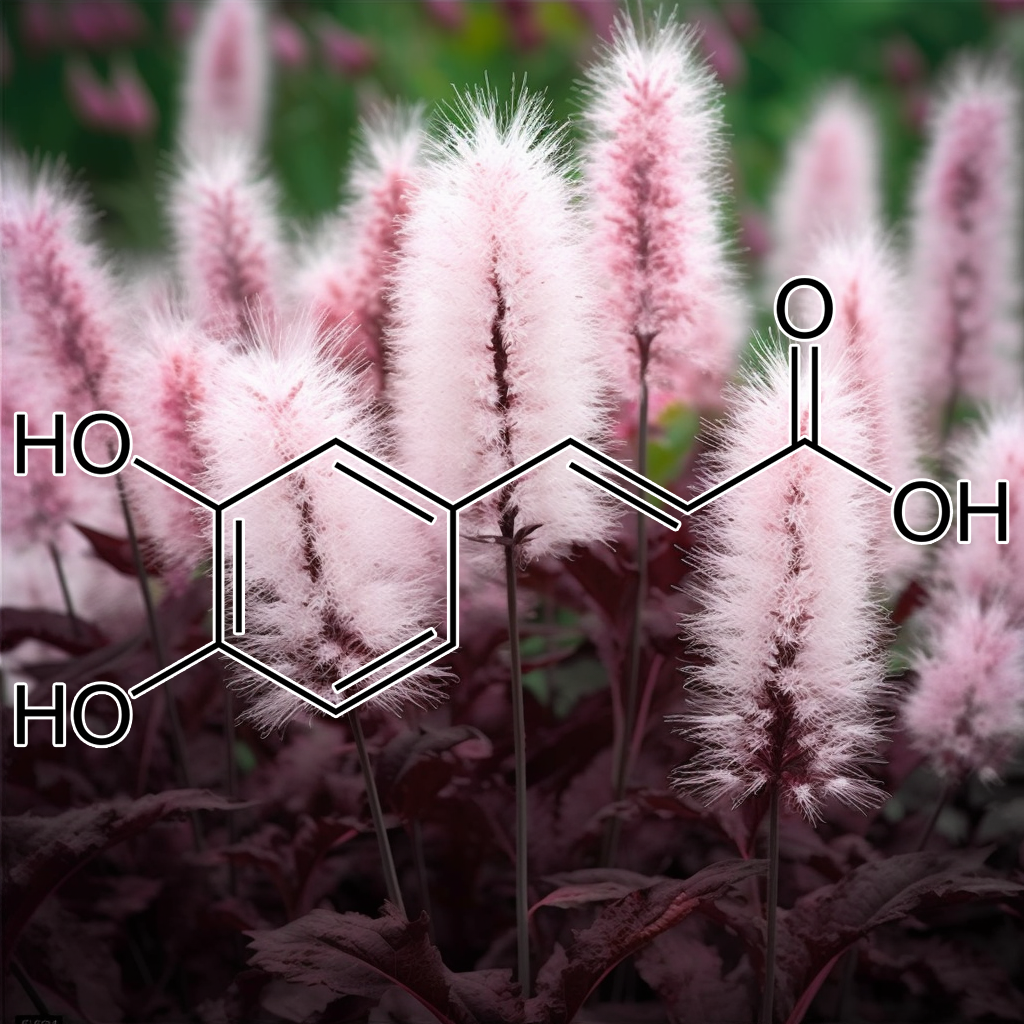


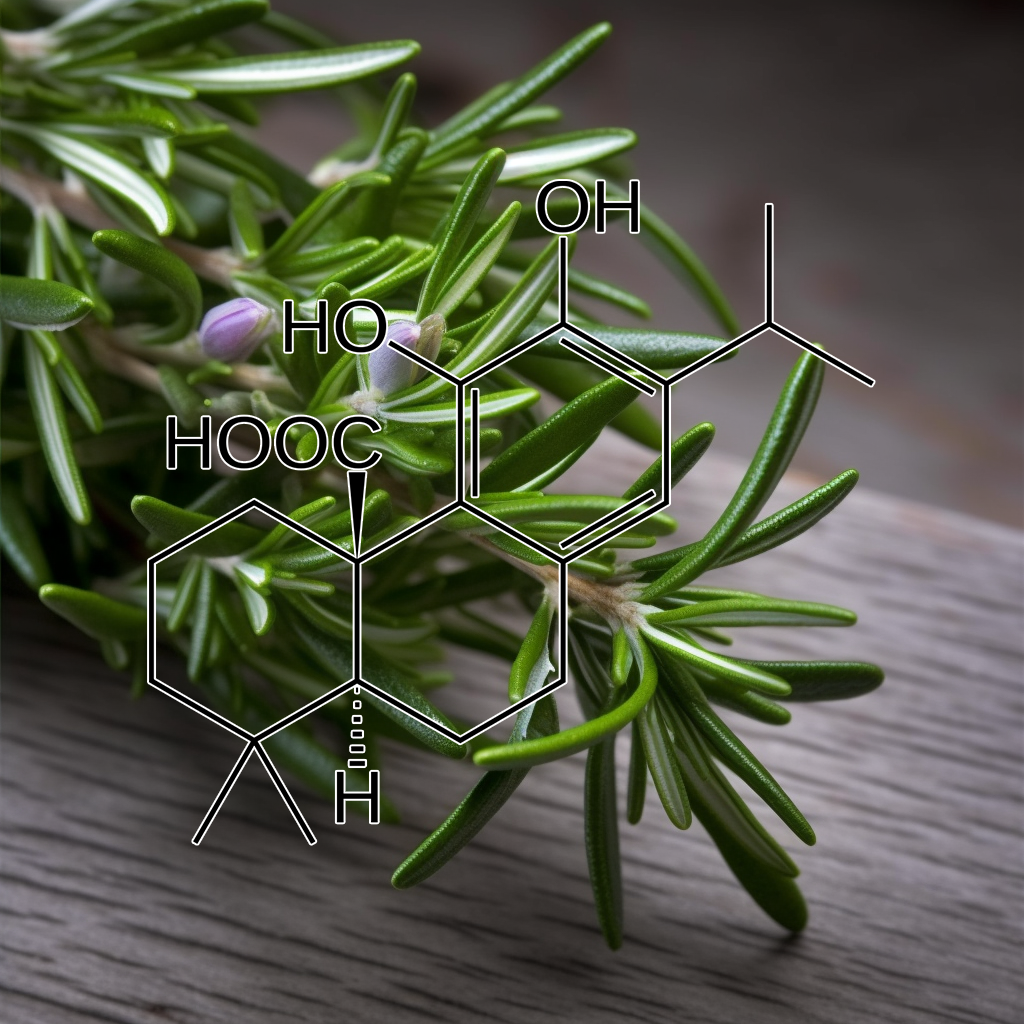


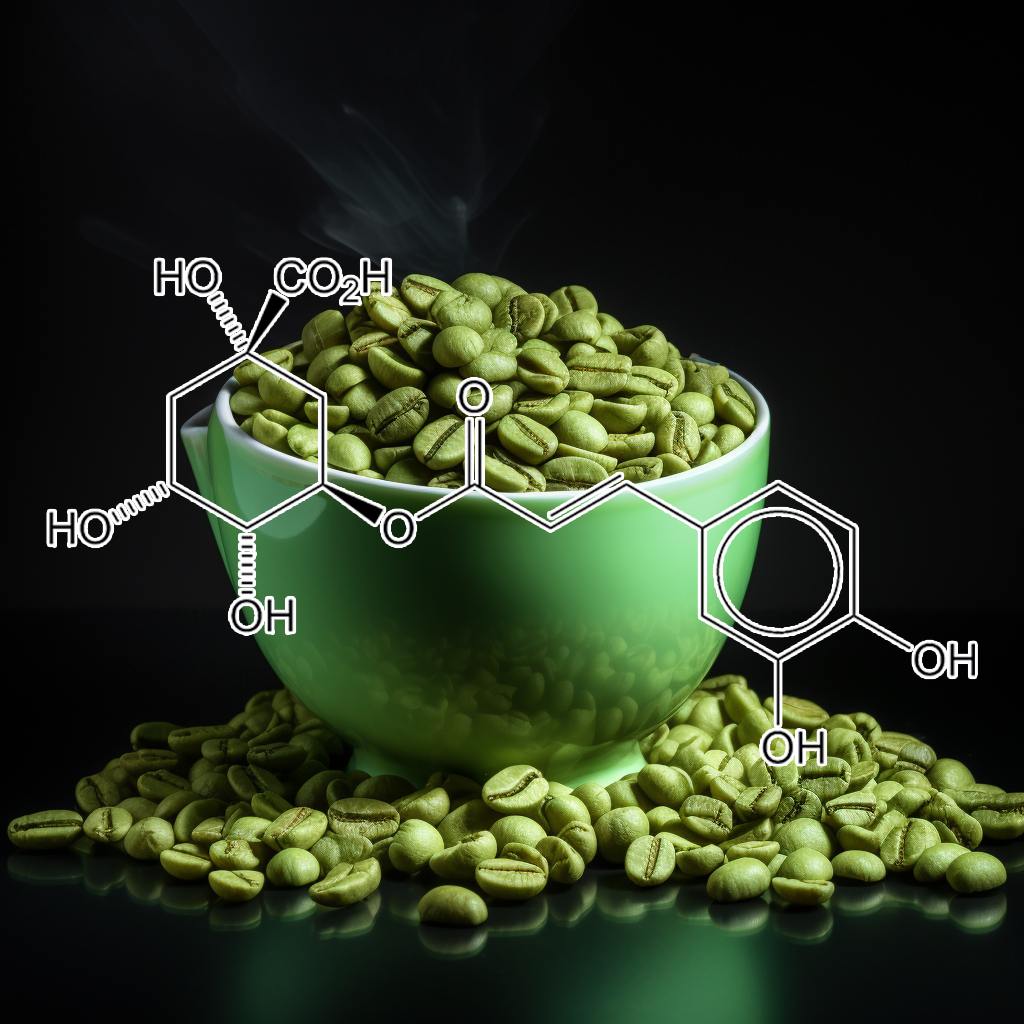




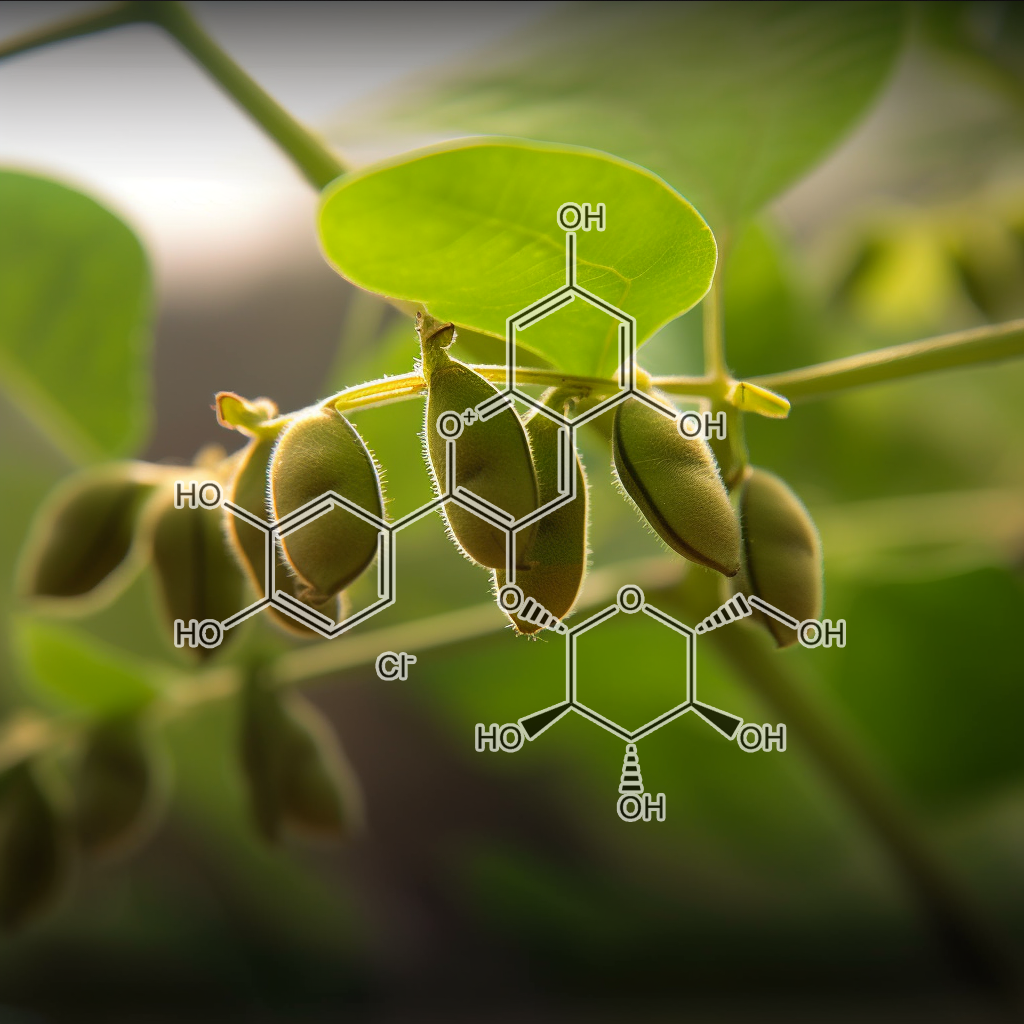









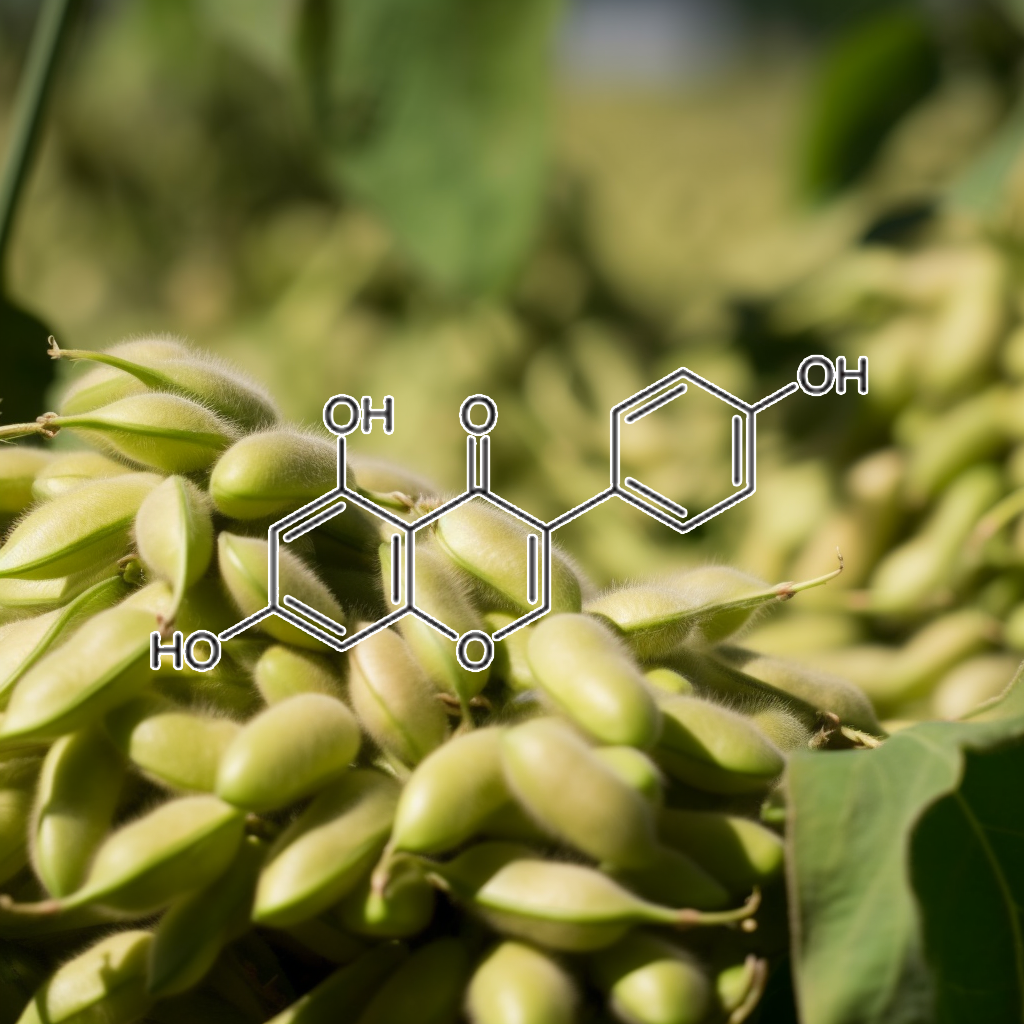

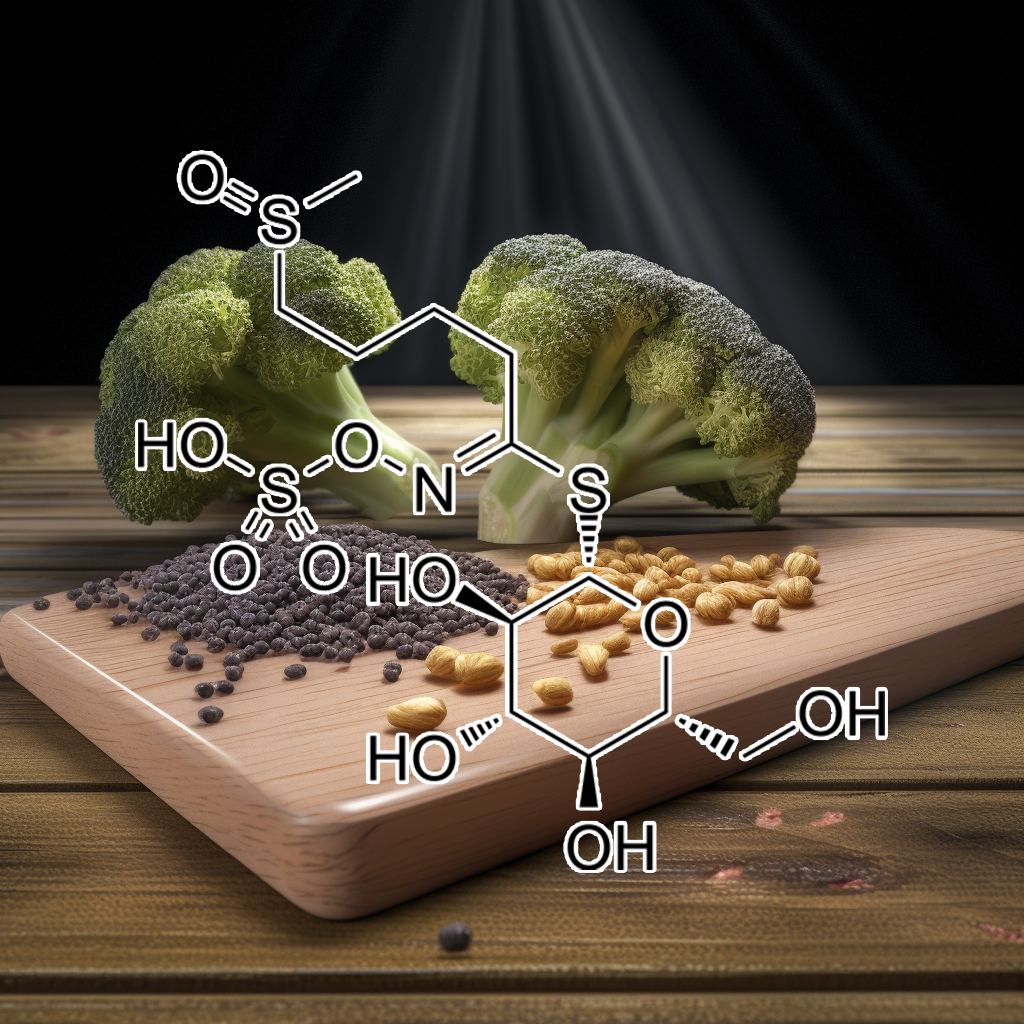





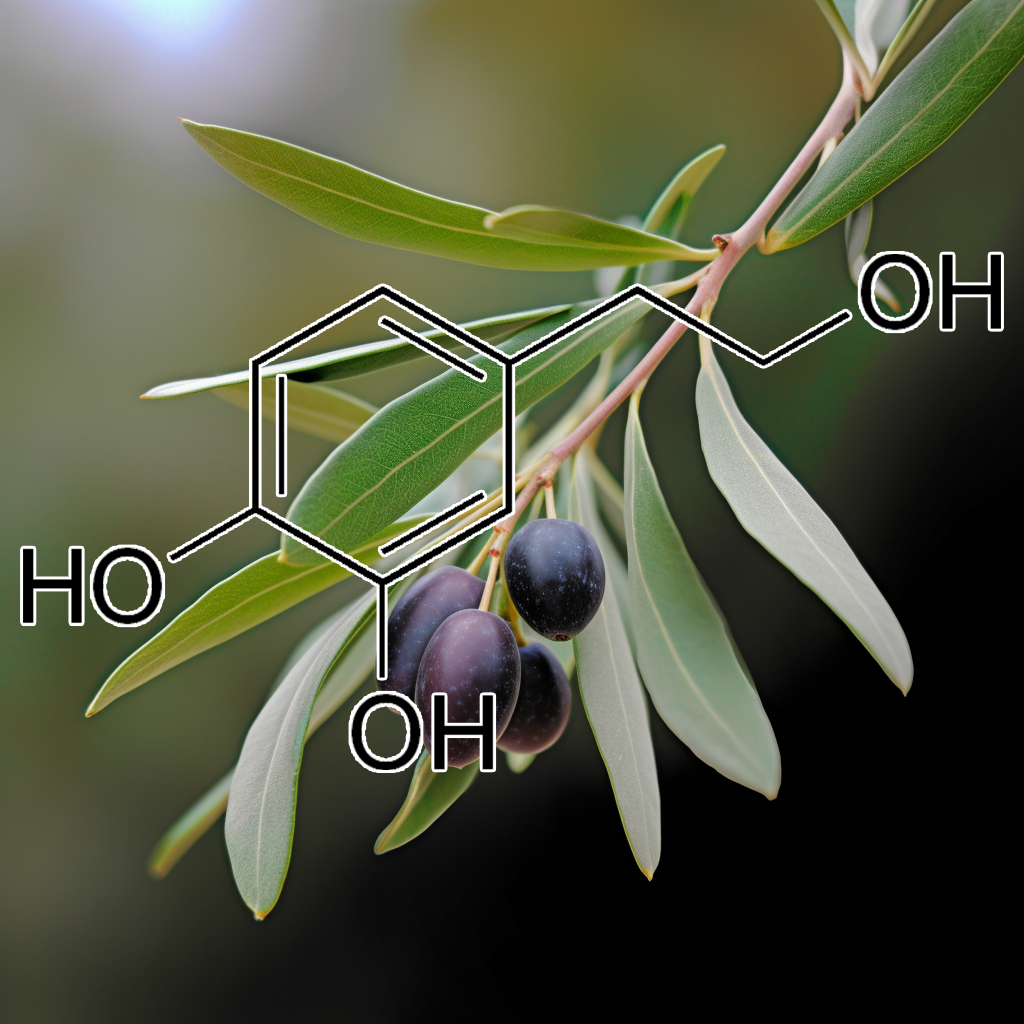










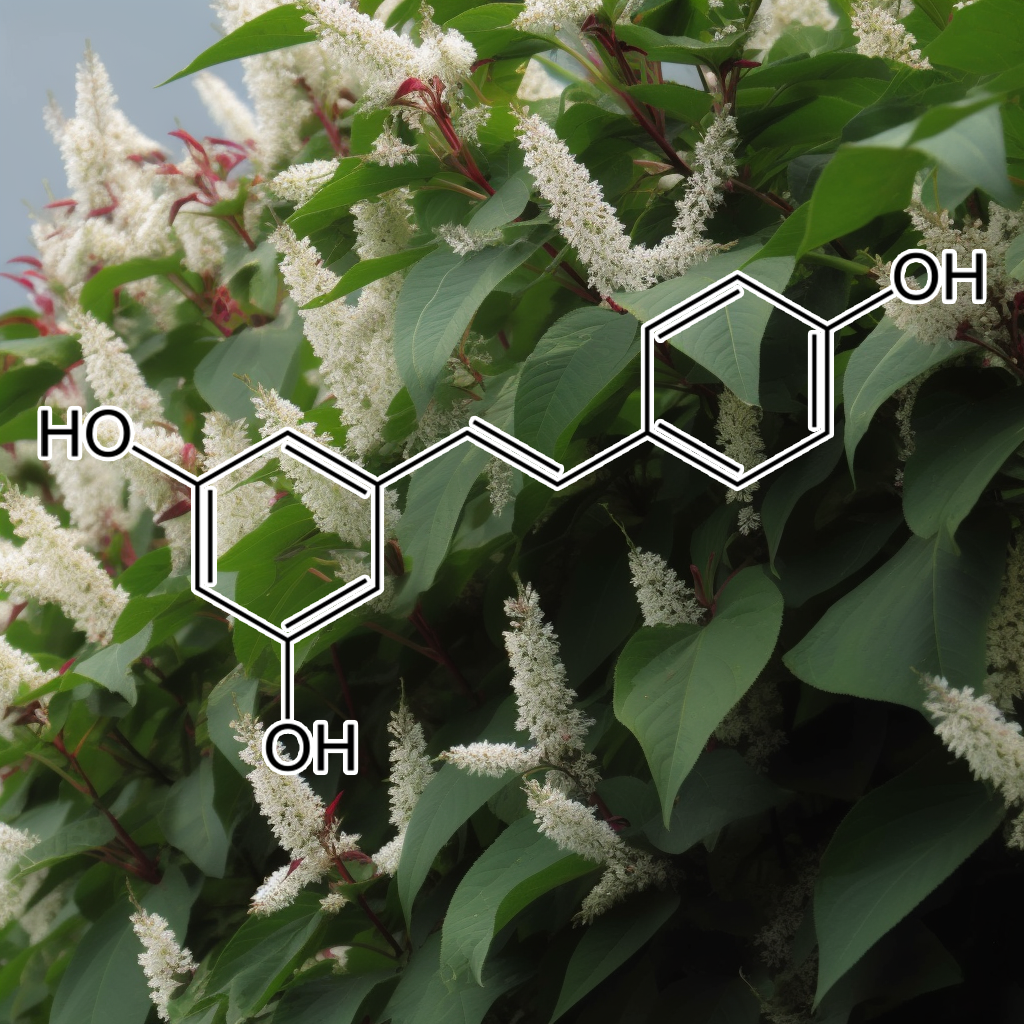

























Heidi –
This blend is my favorite blend! I have a ton of blends from Interstellar! However this blend, NRF2 quickly climbed to the very top after a recent experience I had with it!
I have been dry fasting and rolling 48-72 hour fasts. During one of my dry fasts I added this blend into my liquid restricted fast. So basically I had been dry fasting but adding 1oz of liquid to take my blends. I solely added nrf2 as I had read that it was great for oxidative stress. This blend hit me good. I felt it on a therapeutic level! I felt rejuvenated, uplifted, and almost calling it a spiritual experience! My mind was open, clear, fortified. My body felt whole. I felt “supported”. It was an awesome experience!
With that level of experience I started adding it daily into my routine, and after reading up more about it, I came to the conclusion; what doesn’t it support? As a single blend it has so many benefits it’s hard to say what it does because its range of properties of human molecular support is quite broad, in a good way!!!
I have used this for some neuropathy challenges, hair growth, mental clarity, weight loss, and many others. But it does it all to say the least! It has anti cancerous effects, protects the liver, promotes healthy function, protects against cardiovascular disease, prevention of illness, brain protection, and so much more!
I highly suggest getting NRF2 Activator into your hands! Take it daily. Try it out on a fasted state. The benefits are numerous and the support you feel from it immediately is like no other blend I’ve experienced! This is definitely a top favorite and I won’t be caught without this one in my supply!
Thank you Gavin! For an amazing blend, supporting weight loss, cardiovascular, neurological protection, kidney support, liver support, and reaching my body on a therapeutic molecular level! I felt its benefits from head to toe!! You should definitely give it a try!!
Pablo Arteaga –
4a. NAD+ NRF2 SIRT1, prevent and reverse damage done by free radicals, and maintain basic cellular health with these 3, which are critically interconnected. Take all 3, simultaneously, for best results
4b. Desired Effect: Restore damaged, stressed, oxidized cells & inflamed cells. Actual Effect(G.GEMINI): NAD+, Nrf2, and SIRT1 form a positive feedback loop. Adequate NAD+ levels promote SIRT1 activity. SIRT1 activates Nrf2, leading to increased antioxidant defense. Nrf2 indirectly supports NAD+ production by maintaining cellular health.
4c. Forethought: DNA repair, for damaged inflamed cells, is possible. With these 3 blends, in practice with simple physical exercises, along with restorative practices such as cryo, sauna among other procedures, plus with basic breathing exercises, your full cellular restoration & recuperation is within sight.
Pablo Arteaga –
NAD+ NRF2 & SIRT1 prevent and reverse damage done by free radicals, and aid in maintaining basic cellular health with these 3, which are all critically interconnected. Try all 3, simultaneously, for best results. NRF2 ( a basic leucine zipper (bZIP) protein) and SIRT1 (a deacetylase) are crucial components to the down regulation of oxidative stress, evident through a reduction in muscle inflammation (or KNOTS). Add NAD+ (a coenzyme & an oxidizing agent) which is central to the regulation of energy metabolism, DNA damage repair, gene expression, and oxidative stress response. Thus helping with the healthy maintenance of RNA.
Desired Effect: Restore damaged, stressed, oxidized & inflamed cells. To End the cycle of chronic inflammation (which are a result of free radicals) with NRF2. To combat oxidative stress and inhibit the signaling of inflammatorial pathways with SIRT1. NAD+ for an efficient metabolization of both SIRT1 & NRF2, thus resulting in successful cellular regenerative cycles.
Actual Effect: NAD+, Nrf2, and SIRT1 form a positive feedback loop. Adequate NAD+ levels promote SIRT1 activity. SIRT1 activates Nrf2, leading to increased antioxidant defense. Nrf2 indirectly supports NAD+ production by maintaining cellular health. NRF2 may upregulate antiinflammatory genes, thanks to its transcription factor, which is a DNA-binding domain that allows them to bind to specific DNA sequences, called enhancer or promoter sequences.
Forethought: A profound appreciation for the collective research Gavin and the team have been able to discover and have organized into a physical product. DNA repair, for damaged inflamed cells, is possible. With these 3 blends, in practice with simple physical exercises, along with restorative practices such as cryo, sauna among other procedures, plus with basic breathing exercises, full cellular restoration & recuperation is within reach.