
25 BLEND MEGA COMBO (Save 59%)
February 20, 2019
APRIL SPECIAL! 10 Blend Combo Sampler (Save 56%) $1000 value for $444! Comes with Interstellar Mug!
March 30, 2019SUPER TONIC HAIR 200:1
$275.00
INTRODUCING
INTERSTELLAR BLEND
SUPER TONIC HAIR





200:1 concentration (100 Grams)
Pairs perfectly with
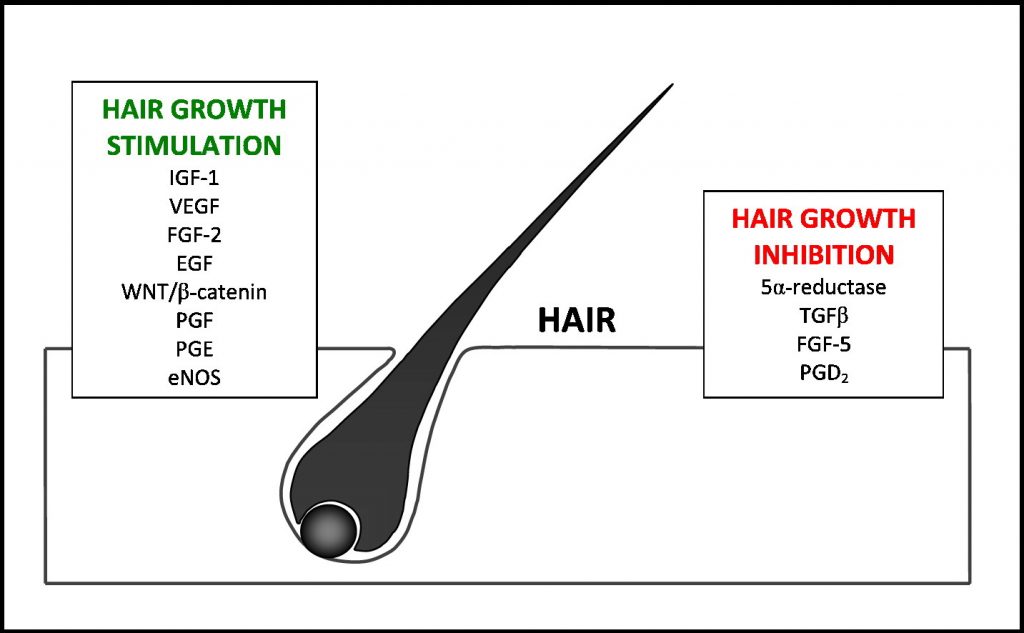
INHIBITS GRAYING
PROMOTES HAIR GROWTH
Directions: Start 1-2 tsp a day for 2 weeks then maintain with 1/4-1/2 tsp daily.
INGREDIENTS: Andrographolide (Andrographis paniculata) • Anthocyanins • Apigenin • Arctigenin (from Fructus Arctii) • Asiasari radix • Astragali radix • Baicalin • Brazilian propolis extract powder • Buxus wallichiana Baill (Buxaceae) • Capparis spinosa • Carnosol • Ceratonia siliqua pod extract • Chrysanthemum zawadskii • Cordycepin • Cranberry peel • Curcumin • Cyanidin • Cycloastragenol • DHEA • Dragon’s Blood from Croton lechleri • Eclipta prostrata • EGCG • Ellagic acid • Emodin • Equisetum arvense • Fermented barley extract • Ferulic acid • Fucoxanthin • Ginseng radix • Glycyrrhiza glabra root extract • Grape seed proanthocyanidin extract • Hesperidin • Hibiscus rosa-sinensis L. flowers • Houttuynia cordata • Hydroxytyrosol • Icariin • Isorhapontigenin • kaempferol • Kolaviron • Kurarinone (From roots of Sophora • Ligustri fructus • Lonicera japonica flower • Luteolin • Metasequoia glyptostroboides leaves &bark extract • Myricetin • N-acetyl- glucosamine • Naringenin • Naringin • Paeoniflorin • Perilla frutescens var. acuta (PFVA) • Pinocembrin • Piper nigrum extract (Piperaceae) • Plectranthus barbatus extract • Polyporus umbellatus • Pomegranate peel extract • Procyanidins from apple peel • Psoralea corylifolia (psoralidin) • Pterostilbene • Quercetin • Radix Angelicae sinensis • Rehmannia glutinosa • Resveratrol • Sanguisorba officinalis • Silibinin • Sophora flavescens root • Tripterygium wilfordii (Celastrol) extract triptolide • Ursolic acid • Urtica dioica
Age‐induced hair greying – the multiple effects of oxidative stress
An obvious sign of ageing is hair greying, or the loss of pigment production and deposition within the hair shafts. Numerous mechanisms, acting at different levels and follicular locations, contribute to hair greying, ranging from melanocyte stem cells defects to follicular melanocyte death. One key issue that is in common to these processes is oxidative damage. At the hair follicle stem cells niche, oxidative stress, accelerated by B‐cell lymphoma 2 gene (BCL‐2) depletion, leads to selective apoptosis and diminution of melanocyte stem cells, reducing the repopulation of newly formed anagen follicles. Melanotic bulbar melanocytes express high levels of BCL‐2 to enable survival from melanogenesis‐ and ultraviolet A (UVA)‐induced reactive oxygen species (ROS) attacks. With ageing, the bulbar melanocyte expression of anti‐oxidant proteins such as BCL‐2, and possibly TRP‐2, is reduced, and the dedicated enzymatic anti‐oxidant defence system throughout the follicle weakens, resulting in enhanced oxidative stress. A marked reduction in catalase expression and activity results in millimolar accumulation of hydrogen peroxide, contributing to bulbar melanocyte malfunction and death. Interestingly, amelanotic melanocytes at the outer root sheath (ORS) are somewhat less affected by these processes and survive for longer time even within the white, ageing hair follicles. Better understanding of the overtime susceptibility of melanocytes to oxidative stress at the different follicular locations might yield clues to possible therapies for the prevention and reversal of hair greying.
Oxidative Stress in Ageing of Hair
Experimental evidence supports the hypothesis that oxidative stress plays a major role in the ageing process. Reactive oxygen species are generated by a multitude of endogenous and environmental challenges. Reactive oxygen species or free radicals are highly reactive molecules that can directly damage cellular structural membranes, lipids, proteins, and DNA. The body possesses endogenous defence mechanisms, such as antioxidative enzymes and non-enzymatic antioxidative molecules, protecting it from free radicals by reducing and neutralizing them. With age, the production of free radicals increases, while the endogenous defence mechanisms decrease. This imbalance leads to the progressive damage of cellular structures, presumably resulting in the ageing phenotype. Ageing of hair manifests as decrease of melanocyte function or graying, and decrease in hair production or alopecia. There is circumstantial evidence that oxidative stress may be a pivotal mechanism contributing to hair graying and hair loss. New insights into the role and prevention of oxidative stress could open new strategies for intervention and reversal of the hair graying process and age-dependent alopecia.
Age-induced hair graying (canities), or the age-induced loss of melanin synthesis and deposition within the hair shafts, is a noticeable and undesired sign of the aging process. Numerous mechanisms contribute to age-induced hair graying, affecting both follicular and stem cell melanocytes and acting at different follicular locations. Many of these processes are induced, directly or indirectly, by oxidative insults and damage. Melanin-producing bulbar melanocytes express high levels of BCL-2 to survive reactive oxygen species (ROS) attacks, which are induced by the melanogenic process itself and by ultraviolet A (UVA) irradiation. With aging, the expression of BCL-2, and possibly of TRP-2, is reduced, and the endogenous, enzymatic antioxidant defense system declines, resulting in greater oxidative stress. In particular, catalase expression and activity are markedly reduced with aging, leading to millimolar accumulation of hydrogen peroxide within the hair follicle and contributing to bulbar melanocyte failure and death. Additionally, exposure of melanocyte stem cells to cumulative oxidative damage, combined with reduced BCL-2 protective levels, results in apoptosis and therefore decreases the number of melanocytes that could repopulate the newly formed anagen follicles. Altogether, oxidative stress may contribute to age-induced hair graying via multiple pathways. Better understanding of the different processes, sources, and types of oxidative stress within the follicular environment, and the different susceptibilities of melanocytes to oxidative stress at the different follicular locations, might yield clues to possible interventions for prevention or reversal of hair graying.
a “free radical theory of graying”
Here we provide unique evidence for oxidative stress induced loss of melanocytes from the human hair follicle during aging. In detail, we show for the first time that:1)a decreased number of viable melanocytes in the aging hair follicle bulge and bulb and an increased incidence of hair bulb melanocyte apoptosis in aging individuals are associated with oxidative stress in the pigmentary unit;2)the aging hair follicle is characterized by the absence of oxidative stress-protectors, such as Bcl-2, and melanocyte growth factors, such as c-Kit;3)a higher frequency of oxidative stress associated mitochondrial DNA damage occurs in graying hair follicles, while unpigmented hair follicles prove to be not “older” than pigmented hair follicles;4)melanocytes of the pigmentary unit are highly and selectively susceptible to exogenous oxidative stress damage.One major route, by which oxidative stress leads to permanent melanocyte damage, appears to pass by the mitochondria, since their DNA is not so well protected as genomic DNA. The accumulation of mutations, therefore, correlates with age and is indicative of general exposure and generation of oxidative stress, (39⤻, 40)⤻caused for example by psychoemotional stress, inflammation, UV-light, and others. In summary, our findings support the proposed hypothesis of a “free radical theory of graying” and suggest that melanocytes in the hair follicle are highly susceptible to endogenous oxidative stress. Exogenous oxidative stress can trigger and hasten this process and provoke permanent damage selectively and prematurely in hair bulb melanocytes.
Humans are social animals that communicate disproportionately via potent genetic signals imbued in the skin and hair, including racial, ethnic, health, gender, and age status. For the vast majority of us, age-related hair pigment loss becomes the inescapable signal of our disappearing youth. The hair follicle (HF) pigmentary unit is a wonderful tissue for studying mechanisms generally regulating aging, often before this becomes evident elsewhere in the body. Given that follicular melanocytes (unlike those in the epidermis) are regulated by the hair growth cycle, this cycle is likely to impact the process of aging in the HF pigmentary unit. The formal identification of melanocyte stem cells in the mouse skin has spurred a flurry of reports on the potential involvement of melanocyte stem cell depletion in hair graying (i.e., canities). Caution is recommended, however, against simple extrapolation of murine data to humans. Regardless, hair graying in both species is likely to involve an age-related imbalance in the tissue’s oxidative stress handling that will impact not only melanogenesis but also melanocyte stem cell and melanocyte homeostasis and survival. There is some emerging evidence that the HF pigmentary unit may have regenerative potential, even after it has begun to produce white hair fibers. It may therefore be feasible to develop strategies to modulate some aging-associated changes to maintain melanin production for longer.
Chronic inflammation induces telomere dysfunction and accelerates ageing
Chronic inflammation is associated with normal and pathological ageing. Here we show that chronic, progressive low-grade inflammation induced by knockout of the nfkb1subunit of the transcription factor NF-κB induces premature ageing in mice. We also show that these mice have reduced regeneration in liver and gut. nfkb1−/−fibroblasts exhibit aggravated cell senescence because of an enhanced autocrine and paracrine feedback through NF-κB, COX-2 and ROS, which stabilizes DNA damage. Preferential accumulation of telomere-dysfunctional senescent cells in nfkb1−/−tissues is blocked by anti-inflammatory or antioxidant treatment of mice, and this rescues tissue regenerative potential. Frequencies of senescent cells in liver and intestinal crypts quantitatively predict mean and maximum lifespan in both short- and long-lived mice cohorts. These data indicate that systemic chronic inflammation can accelerate ageing via ROS-mediated exacerbation of telomere dysfunction and cell senescence in the absence of any other genetic or environmental factor.
Aging is associated with circulating cytokine dysregulation
Aging has a significant impact on the production of circulating cytokines in healthy individuals. The circulating cytokine milieu may contribute to the development of age-restricted conditions. Aging has a significant impact on the production of circulating cytokines. Cytokines dysregulation is demonstrated ex vivo but not after in vitro activation. Pro- and anti-inflammatory cytokines correlate with aging. Th1 cytokines increase whereas Th17 cytokines decrease with age.
Repigmentation and new growth of hairs after anti–interleukin-17 therapy
Repigmentation of hairs is a rare event that has been reported after inflammatory processes, exposure to X-irradiation and psoralen and ultraviolet A, electron beam therapy, and the intake of some drugs.1 We report on a patient with psoriasis who experienced darkening and noticeable increase in scalp hair while he was receiving anti–interleukin (IL)-17 therapy.
Fibroblast growth factor signalling in the hair growth cycle
Using RNA in situ hybridization analysis, we have characterized the expression domains of the four known members of the FGF receptor-tyrosine kinase gene family in the murine hair follicle at various stages of the hair growth cycle. During anagen, we detected Fgfr1 RNA in the dermal papilla, Fgfr2 RNA in hair matrix cells near the dermal papilla, Fgfr3 RNA in pre-cuticle cells in the periphery of the hair bulb, and Fgfr4 RNA in cells in the periphery of the hair bulb and also in the inner and outer root sheath in the lower half of the follicle neck. No RNA expression of these genes was detected during late catagen or telogen. We have previously shown that Fgf5 is expressed in the outer root sheath in the transient portion of the follicle (Hébert et al. [1994] Cell 78:1017-1025). In the present study we have also assayed for the expression of six other members of the FGF ligand gene family, Fgf3, Fgf4, Fgf6, Fgf7, Fgf8, and Fgf9. Among these FGF genes, only Fgf7 was found to be expressed in the hair follicle. Fgf7 RNA is localized to the dermal papilla during anagen, but expression is down-regulated by the late-anagen VI stage. We have also demonstrated that addition of FGF5 protein to the culture medium changes the behavior of dermal papilla cells in vitro, indicating that they are capable of responding to FGF5. Together with previously published data, these results provide a complete analysis of FGF ligand and FGF receptor-tyrosine kinase gene expression in the hair follicle, and suggest that FGF signalling may have several functions in the hair growth cycle
Eclipta prostrata sustains the anagen phase through regulation of FGF-7 & FGF-5
Hair shaft producing cells were proliferated only in the anagen. Many mediators were involved in starting, sustaining and terminating anagen. Among them, we focused on the growth factor FGF-7 and FGF-5. Our results reveal the hair growth enhancing effects of EP were related with the anagen regulating growth factors. mTOR activating the potential of EP in HDPs was signifying that it exerts the positive role in the proliferation of follicular cells during anagen.
FGF5 is a crucial regulator of hair length in humans
Hair length varies dramatically on different body sites and also varies between individuals. Thus, hair length is a quantitative trait, suggesting inherited differences. In this study, we obtained DNA from families segregating excessively long eyelashes consistent with an autosomal recessive trait. We identified mutations in a single gene, fibroblast growth factor 5 (FGF5), which was homozygous in affected family members only. FGF5 has previously been implicated as a regulator of hair lengths in mammals, with mutations resulting in the well-described angoraphenotype. However, until now a human counterpart to this phenotype remained elusive. Here, we present, to our knowledge, the first human counterpart of the angoraphenotype, showing that FGF5 underlies trichomegaly and is a crucial regulator of hair growth in humans.
Oxidative stress management in the hair follicle: Targeting NRF2
Widespread expression of the transcription factor, nuclear factor (erythroid‐derived 2)‐like 2 (NRF2), which maintains redox homeostasis, has recently been identified in the hair follicle (HF). Small molecule activators of NRF2 may therefore be useful in the management of HF pathologies associated with redox imbalance, ranging from HF greying and HF ageing via androgenetic alopecia and alopecia areata to chemotherapy‐induced hair loss. Indeed, NRF2 activation has been shown to prevent peroxide‐induced hair growth inhibition. Multiple parameters can increase the levels of reactive oxygen species in the HF, for example melanogenesis, depilation‐induced trauma, neurogenic and autoimmune inflammation, toxic drugs, environmental stressors such as UV irradiation, genetic defects and aging‐associated mitochondrial dysfunction. In this review, the potential mechanisms whereby NRF2 activation could prove beneficial in treatment of redox‐associated HF disorders are therefore discussed.
The impact of oxidative stress on hair
Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system’s ability to detoxify the reactive intermediates or to repair the resulting damage. Reactive oxygen species or free radicals are highly reactive molecules that can directly damage lipids, proteins, and DNA. They are generated by a multitude of endogenous and environmental challenges, while the body possesses endogenous defense mechanisms. With age, production of free radicals increases, while the endogenous defense mechanisms decrease. This imbalance leads to progressive damage of cellular structures, presumably resulting in the aging phenotype. While the role of oxidative stress has been widely discussed in skin aging, little focus has been placed on its impact on hair condition. Moreover, most literature on age‐related hair changes focuses on alopecia, but it is equally important that the hair fibers that emerge from the scalp exhibit significant age‐related changes that have equal impact on the overall cosmetic properties of hair. Sources of oxidative stress with impact on the pre‐emerging fiber include: oxidative metabolism, smoking, UVR, and inflammation from microbial, pollutant, or irritant origins. Sources of oxidative stress with impact on the post‐emerging fiber include: UVR (enhanced by copper), chemical insults, and oxidized scalp lipids. The role of the dermatologist is recognition and treatment of pre‐ and post‐emerging factors for lifetime scalp and hair health.
Repigmentation of hair following adalimumab therapy
Repigmentation of canities, or age-related grey or white hair, is a rare occurrence. Generalized repigmentation of grey-white hair has been reported following inflammatory processes,[1] and heterochromia (localized patches of hair repigmentation) is even more unusual, reported in association with medication use and malignancy.Tumor necrosis factor (TNF) inhibitors are increasingly utilized medications for inflammatory disorders, including psoriasis, rheumatoid arthritis, and inflammatory bowel disease. Hair loss, or alopecia, has been described among the side effects of these medications,[2] but changes in hair pigmentation in association with this class of drugs have not previously been reported. We describe a patient with hair repigmentation associated with adalimumab therapy.
A Comment on the Science of Hair Aging
In contrast to the skin, aging of the hair has seemingly only recently found the attention of dermatological meetings, mainly promoted by the cosmetic industry for marketing purposes. In fact, basic scientists interested in the biology of hair growth and pigmentation have for some time already exposed the hair follicle as a highly accessible model with unique opportunities for the study of age-related effects. As a result, the science of hair aging focuses on two main streams of interest: the esthetic problem of aging hair and its management, in terms of age-related effects on hair color, quantity, and quality; and the biological problem of aging hair, in terms of microscopic, biochemical, and molecular changes underlying the aging process. Ultimately, the aim of hair anti-aging is to delay, lessen, or reverse the effects of aging on hair. According to the complex nature of the aging process, the treatment for lifetime scalp and hair health has to be holistic to include the multitude of contributing factors in a polyhedral and patient-specific manner. It comprises both medical treatments and hair cosmetics. Accordingly, the discovery of pharmacological targets and the development of safe and effective drugs for treatment of hair loss indicate strategies of the drug industry for maintenance of hair growth and quantity, while the hair care industry has become capable of delivering active compounds directed toward meeting the consumer demand for maintenance of hair cosmesis and quality. “Where there’s life, there’s hope” (Ecclesiastes 9:3-5).
Three Streams for the Mechanism of Hair Graying
Hair graying is an obvious sign of human aging. Although graying has been investigated extensively, the mechanism remains unclear. Here, we reviewed previous studies on the mechanism of graying and seek to offer some new insights. The traditional view is that hair graying is caused by exhaustion of the pigmentary potential of the melanocytes of hair bulbs. Melanocyte dysfunction may be attributable to the effects of toxic reactive oxygen species on melanocyte nuclei and mitochondria. A recent study suggests that bulge melanocyte stem cells (MSCs) are the key cells in play. Graying may be caused by defective MSC self-maintenance, not by any deficiency in bulbar melanocytes. Our previous study suggested that graying may be principally attributable to active hair growth. Active hair growth may produce oxidative or genotoxic stress in hair bulge. These internal stress may cause eventually depletion of MSC in the hair follicles. Taken together, hair graying may be caused by MSC depletion by genotoxic stress in the hair bulge. Hair graying may also be sometimes caused by dysfunction of the melanocytes by oxidative stress in the hair bulb. In addition, hair graying may be attributable to MSC depletion by active hair growth.
Pharmacologic interventions in aging hair
The appearance of hair plays an important role in people’s overall physical appearance and self-perception. With today’s increasing life-expectations, the desire to look youthful plays a bigger role than ever. The hair care industry has become aware of this and is delivering active products directed towards meeting this consumer demand. The discovery of pharmacological targets and the development of safe and effective drugs also indicate strategies of the drug industry for maintenance of healthy and beautiful hair. Hair aging comprises weathering of the hair shaft, decrease of melanocyte function, and decrease in hair production. The scalp is subject to intrinsic and extrinsic aging. Intrinsic factors are related to individual genetic and epigenetic mechanisms with interindividual variation: prototypes are familial premature graying, and androgenetic alopecia. Currently available pharmacologic treatment modalities with proven efficacy for treatment of androgenetic alopecia are topical minoxidil and oral finasteride. Extrinsic factors include ultraviolet radiation and air pollution. Experimental evidence supports the hypothesis that oxidative stress also plays a role in hair aging. Topical anti-aging compounds include photoprotectors and antioxidants. In the absence of another way to reverse hair graying, hair colorants remain the mainstay of recovering lost hair color. Topical liposome targeting for melanins, genes, and proteins selectively to hair follicles are currently under investigation.
Hair growth inhibition by psychoemotional stress
Stress has long been discussed controversially as a cause of hair loss. However, solid proof of stress‐induced hair growth inhibition had long been missing. If psychoemotional stress can affect hair growth, this must be mediated via definable neurorendocrine and/or neuroimmunological signaling pathways. Revisiting and up‐dating relevant background data on neural mechanisms of hair growth control, we sketch essentials of hair follicle (HF) neurobiology and discuss the modulation of murine hair growth by neuropeptides, neurotransmitters, neurotrophins, and mast cells. Exploiting an established mouse model for stress, we summarize recent evidence that sonic stress triggers a cascade of molecular events including plasticity of the peptidergic peri‐ and interfollicular innervation and neuroimmune crosstalk. Substance P (SP) and NGF (nerve growth factor) are recruited as key mediators of stress‐induced hair growth‐inhibitory effects. These effects include perifollicular neurogenic inflammation, HF keratinocyte apoptosis, inhibition of proliferation within the HF epithelium, and premature HF regression (catagen induction). Intriguingly, most of these effects can be abrogated by treatment of stressed mice with SP‐receptor neurokinin‐1 receptor (NK‐1) antagonists or NGF‐neutralizing antibodies – as well as, surprisingly, by topical minoxidil. Thus there is now solid in vivo‐evidence for the existence of a defined brain‐ HF axis. This axis can be utilized by psychoemotional and other stressors to prematurely terminate hair growth. Stress‐induced hair growth inhibition can therefore serve as a highly instructive model for exploring the brain‐skin connection and provides a unique experimental model for dissecting general principles of skin neuroendocrinology and neuroimmunology well beyond the HF.
Hair cycle and hair pigmentation ASSOCIATED WITH AGING
The tight coupling of hair follicle melanogenesis to the hair growth cycle dramatically distinguishes follicular melanogenesis from the continuous melanogenesis of the epidermis. Cyclic re-construction of an intact hair follicle pigmentary unit occurs optimally in all scalp hair follicles during only the first 10 hair cycles, i.e. by approximately 40 years of age. Thereafter there appears to be a genetically regulated exhaustion of the pigmentary potential of each individual hair follicle leading to the formation of true gray and white hair. Pigment dilution results primarily from a reduction in tyrosinase activity within hair bulbar melanocytes. Thereafter, sub-optimal melanocyte–cortical keratinocyte interactions, and defective migration of melanocytes from a reservoir in the upper outer root sheath to the pigment-permitting microenvironment close to the follicular papilla of the hair bulb, will all disrupt normal function of the pigmentary unit. Evidence from studies on epidermal melanocyte aging suggest that reactive oxygen species-mediated damage to nuclear and mitochondrial DNA may lead to mutation accumulation in bulbar melanocytes. Parallel dysregulation of anti-oxidant mechanisms or pro/anti-apoptotic factors is also likely to occur within the cells. Pigment loss in canities may also affect keratinocyte proliferation and differentiation, providing the tantalizing suggestion that melanocytes in the hair follicle contribute far more that packages of pigment alone. Here, we review the current state of knowledge of the development, regulation and control of the aging human hair follicle pigmentary system in relation with hair cycling. The exploitation of recently available methodologies to manipulate hair follicle melanocytes in vitro, and the observations that melanocytes remain in senile white hair follicles that can be induced to pigment in culture, raises the possibility of someday reversing canities. The perspective of rejuvenation of the whole hair follicle apparatus are still part of the dream but optimising its functional properties is clinically relevant and is close to reality. Finally as hair color influences its visibility when optical methods such as scalp photography are used to count hair fibers, the attention is drawn to possible interpretations of statistically significant changes in visible hair. Such changes may not exclusively be related to improved hair growth itself but also to changes in natural hair color that makes the hair more visible with the method used to count hairs.
Human hair pigmentation – biological aspects
Skin and hair colour contribute significantly to our overall visual appearance and to social/sexual communication. Despite their shared origins in the embryologic neural crest, the hair follicle and epidermal pigmentary units occupy distinct, although open, cutaneous compartments. They can be distinguished principally on the basis of the former’s stringent coupling to the hair growth cycle compared with the latter’s continuous melanogenesis. The biosynthesis of melanin and its subsequent transfer from melanocyte to hair bulb keratinocytes depend on the availability of melanin precursors and on a raft of signal transduction pathways that are both highly complex and commonly redundant. These signalling pathways can be both dependent and independent of receptors, act through auto‐, para‐ or intracrine mechanisms and can be modified by hormonal signals. Despite many shared features, follicular melanocytes appear to be more sensitive than epidermal melanocytes to ageing influences. This can be seen most dramatically in hair greying/canities and this is likely to reflect significant differences in the epidermal and follicular microenvironments. The hair follicle pigmentary unit may also serve as an important environmental sensor, whereby hair pigment contributes to the rapid excretion of heavy metals, chemicals and toxins from the body by their selective binding to melanin; rendering the hair fibre a useful barometer of exposures. The recent availability of advanced cell culture methodologies for isolated hair follicle melanocytes and for intact anagen hair follicle organ culture should provide the research tools necessary to elucidate the regulatory mechanisms of hair follicle pigmentation. In the longer term, it may be feasible to develop hair colour modifiers of a biological nature to accompany those based on chemicals.
Histopathology of aging of the hair follicle
Hair follicles experience several changes with aging, the most noticeable of which is graying of the hair shaft due to loss of melanin. Additional changes in the diameter and length of the hair have contributed to the concept of senescent alopecia, which is different from androgenetic alopecia according to most. Graying happens in most individuals, although in different grades and starting at different ages. It is related to a decrease in the number and activity of the melanocytes of the hair bulb, which eventually completely disappear from the bulb of the white hair. Residual non‐active melanocytes remain in the outer root sheath and in the bulge, which allows for repigmentation of the hair under certain stimuli or conditions.
the etiologies, clinical characteristics, and treatment of grey hair
Hair pigmentation is regulated by follicular melanogenesis, in which the process consists of melanin formation and transfer to keratinocytes in the hair shaft. Human hair follicles contain two types of melanin: the brown‐black eumelanin and yellow‐red pheomelanin. Eumelanin is commonly present in black and brown hair while pheomelanin is found in auburn and blonde hair. Hair follicle melanogenesis is under cyclical control and is concurrently coupled to hair growth. Many factors including intrinsic and extrinsic factors affect the follicular melanogenesis. Though many studies have been conducted to identify the pathogenesis and regulation of hair pigmentation, the etiology of canities and hair pigmentation is still unclear. The pathogenesis of canities or gray hair is believed to occur either from insufficient melanin formation due to melanocyte degeneration or a defect in melanosomal transfer. Canities is an aging sign which often interferes with one’s socio‐cultural adjustment. On the other hand, premature canities correlate with diseases such as osteopenia and cardiovascular disease. Risk factors associated with canities are not only genetic but also external causes. For example, smoking, alcohol consumption, and stress are among the most common factors. Camouflage techniques are still used as the primary treatment of canities. Further treatments for canities are being developed to achieve the true reversal of hair pigmentation.
Aging of the Hair Follicle Pigmentation System
Skin and hair phenotypes are powerful cues in human communication. They impart much information, not least about our racial, ethnic, health, gender and age status. In the case of the latter parameter, we experience significant change in pigmentation in our journey from birth to puberty and through to young adulthood, middle age and beyond. The hair follicle pigmentary unit is perhaps one of our most visible, accessible and potent aging sensors, with marked dilution of pigment intensity occurring long before even subtle changes are seen in the epidermis. This dichotomy is of interest as both skin compartments contain melanocyte subpopulations of similar embryologic (i.e., neural crest) origin. Research groups are actively pursuing the study of the differential aging of melanocytes in the hair bulb versus the epidermis and in particular are examining whether this is in part linked to the stringent coupling of follicular melanocytes to the hair growth cycle. Whether some follicular melanocyte subpopulations are affected, like epidermal melanocytes, by UV irradiation is not yet clear. A particular target of research into hair graying or canities is the nature of the melanocyte stem compartment and whether this is depleted due to reactive oxygen species-associated damage, coupled with an impaired antioxidant status, and a failure of melanocyte stem cell renewal. Over the last few years, we and others have developed advanced in vitro models and assay systems for isolated hair follicle melanocytes and for intact anagen hair follicle organ culture which may provide research tools to elucidate the regulatory mechanisms of hair follicle pigmentation. Long term, it may be feasible to develop strategies to modulate some of these aging-associated changes in the hair follicle that impinge particularly on the melanocyte populations.
Autocrine and paracrine factors are produced by balding dermal papilla (DP) cells following dihydrotestosterone (DHT)-driven alterations and are believed to be key factors involved in male pattern baldness. Herein we report that the IL-6 is upregulated in balding DP cells compared with non-balding DP cells. IL-6 was upregulated 3 hours after 10-100 nM DHT treatment, and ELISA showed that IL-6 was secreted from balding DP cells in response to DHT. IL-6 receptor (IL-6R) and glycoprotein 130 (gp130) were expressed in follicular keratinocytes, including matrix cells. Recombinant human IL-6 (rhIL-6) inhibited hair shaft elongation and suppressed proliferation of matrix cells in cultured human hair follicles. Moreover, rhIL-6 injection into the hypodermis of mice during anagen caused premature onset of catagen. Taken together, our data strongly suggest that DHT-inducible IL-6 inhibits hair growth as a paracrine mediator from the DP.
Cytokines and Other Mediators in Alopecia Areata
Alopecia areata, a disease of the hair follicles with multifactorial etiology and a strong component of autoimmune origin, has been extensively studied as far as the role of several cytokines is concerned. So far, IFN-, interleukins, TNF-, are cytokines that are well known to play a major role in the pathogenesis of the disease, while several studies have shown that many more pathways exist. Among them, MIG, IP-10, BAFF, HLA antigens, MIG, as well as stress hormones are implicated in disease onset and activity. Within the scope of this paper, the authors attempt to shed light upon the complexity of alopecia areata underlying mechanisms and indicate pathways that may suggest future treatments.
INGREDIENTS & SCIENCE

Andrographis Paniculata
- Antioxidant and Anti-Inflammatory Activities of the Plant Andrographis Paniculata Nees
- Anti-inflammatory Activity of New Compounds from Andrographis paniculata by NF-κB Transactivation Inhibition
- Inhibitory effect of andrographolidefrom Andrographis paniculata on PAF-induced platelet aggregation
- Study of anti-inflammatory activities of the pure compounds from Andrographis paniculata (burm.f.) Nees and their effects on gene expression
- Effect of an extract of Andrographis paniculata leaves on inflammatory and allergic mediators in vitro
- AP-1/IRF-3 Targeted Anti-Inflammatory Activity of Andrographolide Isolated from Andrographis paniculata
- Antiangiogenic activity of Andrographis paniculata extract and andrographolide
- In vitro modulation of LPS/calcimycin induced inflammatory and allergic mediators by pure compounds of Andrographis paniculata(King of bitters) extract
- Inhibitory Effects of Ethyl Acetate Extract of Andrographis paniculata on NF-κB Trans-Activation Activity and LPS-Induced Acute Inflammation in Mice
- Andrograpanin, a compound isolated from anti‐inflammatorytraditional Chinese medicine Andrographis paniculata, enhances chemokine SDF‐1α‐induced leukocytes chemotaxis
- An in vitro study of anti-inflammatory activityof standardised Andrographis paniculata extractsand pure andrographolide
- Andrograpanin, isolated from Andrographis paniculata, exhibits anti-inflammatory property in lipopolysaccharide-induced macrophage cells through down-regulating the p38 MAPKs signaling pathways
- Review on Liver Inflammation and Antiinflammatory Activity of Andrographis paniculata for Hepatoprotection
- Effect of noni (Morinda citrifolia ) and fahtalaijons (Andrographis paniculata) on pigmentation and phagocytosis in goldfish (Carasius auratus)
- 5α-reductase inhibition and hair growth promotion of some Thai plants traditionally used for hair treatment
- The anti-inflammatory effect of Andrographis paniculata (Burm. f.) Nees on pelvic inflammatory disease in rats through down-regulation of the NF-κB pathway
- Antioxidant, antinociceptive and anti-inflammatory properties of the aqueous and ethanolic leaf extracts of Andrographis paniculata in some laboratory animals
- Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities
- Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata
- Antioxidant and gastroprotective activities of Andrographis paniculata (Hempedu Bumi) in Sprague Dawley rats
- In Vitro Comparative Evaluation of Non-Leaves and Leaves Extracts of Andrographis paniculata on Modulation of Inflammatory Mediators
- Protective Effects of Andrographis paniculata Extract and Pure Andrographolide Against Chronic Stress-Triggered Pathologies in Rats
- Andrographis paniculata Downregulates Proinflammatory Cytokine Production and Augments Cell Mediated Immune Response in Metastatic Tumor-Bearing Mice
- Rapid extraction of andrographolide from Andrographis paniculataNees by three phase partitioning and determination of its antioxidant activity
- Andrographis paniculata(Burm. f.) Wall. ex Nees (kalmegh), a traditional hepatoprotective drug from India
- ANDROGRAPHIS PANICULATA AND ITS BIOACTIVE PHYTOCHEMICAL CONSTITUENTS FOR OXIDATIVE DAMAGE: A SYSTEMIC REVIEW
- Andrographolide, a major component of Andrographis paniculata leaves, has the neuroprotective effectson glutamate-induced HT22 cell death
- Study on the pharmacokinetics of Andrographis paniculata Nees’s anti-inflammatory effect
- Hepatoprotective Effect of the Aqueous Leaf Extract of Andrographis paniculataNees Against Carbon Tetrachloride – Induced Hepatotoxicity in Rats
- Water Fraction Of Sambiloto (Andrographis Paniculata Nees) Ethanol Extract Efficacy In Inducing The Number Of Macrophage, Neutrophil, And The Level Of TNF-α On Wistar Rats
- Pengaruh ekstrak air herba sambiloto (andrographis paniculata) dan daun salam (syzygium polyanthum) terhadap jumlah tnf-α, makrofag dan neutrofil pada tikus yang diinduksi aloksan
- Effect of Andrographis Paniculata to the Expression of IL-6, IL-17, IL-10, TGFß, and the Ratio of Treg
- Effect of Andrographis Paniculata to the Expression of IL-6, IL-17, IL-10, TGFβ, and the Ratio of Treg/ Th17 in Sprague Dawley Rats with Atherosclerosis Diet an dCigarette Smoke

- Application of Anthocyanins from Blackcurrant (Ribes nigrum L.) Fruit Waste as Renewable Hair Dyes
- Investigation on the dyeing power of some organic natural compounds for a green approach to hair dyeing
- Tart cherry anthocyanins suppress inflammation-induced pain behavior in rat
- Protective Effects of Anthocyanins from Blackberry in a Rat Model of Acute Lung Inflammation
- Anthocyanins from black soybean seed coats stimulate wound healing in fibroblasts and keratinocytes and prevent inflammation in endothelial cells
- Intakes of Anthocyanins and Flavones Are Associated with Biomarkers of Insulin Resistance and Inflammation in Women
- Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: a double-blind randomized placebo-controlled crossover study
- Inhibitory Effects of Wild Blueberry Anthocyanins and Other Flavonoids on Biomarkers of Acute and Chronic Inflammation in Vitro
- Anthocyanins and proanthocyanidins from blueberry–blackberry fermented beverages inhibit markers of inflammation in macrophages and carbohydrate‐utilizing enzymes in vitro
- Purple corn anthocyanins dampened high-glucose-induced mesangial fibrosis and inflammation: possible renoprotective role in diabetic nephropathy
- Synergistic inhibition of interleukin-6 production in adipose stem cells by tart cherry anthocyanins and atorvastatin
- Flavan‐3‐ols, anthocyanins, and inflammation
- Non-anthocyanin phenolics in cherry (Prunus avium L.) modulate IL-6, liver lipids and expression of PPARδ and LXRs in obese diabetic (db/db) mice
- Bilberry-Derived Anthocyanins Modulate Cytokine Expression in the Intestine of Patients with Ulcerative Colitis
- Bilberry-Derived Anthocyanins Prevent IFN-γ-Induced Pro-Inflammatory Signalling and Cytokine Secretion in Human THP-1 Monocytic Cells
- Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress
- The role of anthocyanins as an antioxidant under oxidative stress in rats
- Anthocyanins Induce the Activation of Phase II Enzymes through the Antioxidant Response Element Pathway against Oxidative Stress-Induced Apoptosis
- Strawberry and Its Anthocyanins Reduce Oxidative Stress-Induced Apoptosis in PC12 Cells
- Preventive Effects of Dietary Cabbage Acylated Anthocyanins on Paraquat-induced Oxidative Stress in Rats
- Anthocyanins from Chinese Bayberry Extract Protect β Cells from Oxidative Stress-Mediated Injury via HO-1 Upregulation
- Effects of Anthocyanins on Psychological Stress-Induced Oxidative Stress and Neurotransmitter Status
- Anthocyanins Reversed D-Galactose-Induced Oxidative Stress and Neuroinflammation Mediated Cognitive Impairment in Adult Rats
- Cranberry anthocyanin extract prolongs lifespan of fruit flies
- Dietary Anthocyanins against Obesity and Inflammation
- Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf2/HO-1 signaling
- Anthocyanins from black soybean inhibit Helicobacter pylori‐induced inflammation in human gastric epithelial AGS cells
- Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model
- Anthocyanins inhibit high-glucose-induced cholesterol accumulation and inflammation by activating LXRα pathway in HK-2 cells
- Purple carrot anthocyaninssuppress lipopolysaccharide-induced inflammation in the co-culture of intestinal Caco-2 and macrophage RAW264.7 cells
- Blueberry anthocyanins ameliorate cyclophosphamide-induced liver damage in rats by reducing inflammation and apoptosis
- Anthocyanins and their physiologically relevant metabolites alter the expression of IL‐6 and VCAM‐1 in CD40L and oxidized LDL challenged vascular endothelial cells
- AnthocyaninExtracted from Black Soybean Seed Coats Prevents Autoimmune Arthritis by Suppressing the Development of Th17 Cells and Synthesis of Proinflammatory Cytokines by Such Cells, via Inhibition of NF-κB

- Inhibitory Effect of Apigenin, a Plant Flavonoid, on Epidermal Ornithine Decarboxylase and Skin TumorPromotion in Mice
- Inhibition of ultraviolet light induced skin carcinogenesis in SKH-1 mice by apigenin, a plant flavonoid.
- Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes
- Skin anti-inflammatory activity of apigenin-7-glucoside in rats.
- Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation
- Src kinase is a direct target of apigenin against UVB-induced skin inflammation
- Apigenin Reactivates Nrf2 Anti-oxidative Stress Signaling in Mouse Skin Epidermal JB6 P + Cells Through Epigenetics Modifications
- In Vivo and In Vitro Percutaneous Absorption of Cancer Preventive Flavonoid Apigenin in Different Vehicles in Mouse Skin
- Inhibition of mTOR by apigenin in UVB-irradiated keratinocytes: A new implication of skincancer prevention
- Efficacy of PLGA-loaded apigenin nanoparticles in Benzo[a]pyrene and ultraviolet-B induced skin cancer of mice: Mitochondria mediated apoptotic signalling cascades
- Dietary apigenin attenuates the development of atopic dermatitis-like skin lesions in NC/Nga mice
- Strategic formulation of apigenin-loaded PLGA nanoparticles for intracellular trafficking, DNA targeting and improved therapeutic effects in skin melanoma in vitro
- Influence of Vehicle, Distant Topical Delivery, and Biotransformation on the Chemopreventive Activity of Apigenin, a Plant Flavonoid, in Mouse Skin
- Anti-inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin
- Anti-Inflammatory Effects of Apigenin in Lipopolysaccharide-Induced Inflammatory in Acute Lung Injury by Suppressing COX-2 and NF-kB Pathway
- Decreased pro-inflammatory cytokine production by LPS-stimulated PBMC upon in vitro incubation with the flavonoids apigenin, luteolin or chrysin, due to selective elimination of monocytes/macrophages
- Anti-inflammatory mechanisms of apigenin: inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules
- Apigenin inhibits allergen-induced airway inflammation and switches immune response in a murine model of asthma
- Dietary phytophenols curcumin, naringenin and apigenin reduce infection-induced inflammatory and contractile pathways in human placenta, foetal membranes and myometrium
- Apigenin inhibits release of inflammatory mediators by blocking the NF-κB activation pathways in the HMC-1 cells
- Apigenin Blocks Lipopolysaccharide-Induced Lethality In Vivo and ProinflammatoryCytokines Expression by Inactivating NF-κB through the Suppression of p65 Phosphorylation
- Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin (chamomile), ginsenoside Rb1 (ginseng) and parthenolide (feverfew)
- Dietary Apigenin Suppresses IgE and Inflammatory Cytokines Production in C57BL/6N Mice
- Apigenin inhibits PMA-induced expression of pro-inflammatory cytokines and AP-1 factors in A549 cells
- Apigenin Attenuates Experimental Autoimmune Myocarditis by Modulating Th1/Th2 Cytokine Balance in Mice
- Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects
- Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress
- Apigenin inhibits oxidative stress‐induced macromolecular damage in N‐nitrosodiethylamine (NDEA)‐induced hepatocellular carcinogenesis in Wistar albino rats
- Oxidative stress triggered by naturally occurring flavone apigenin results in senescence and chemotherapeutic effect in human colorectal cancer cells
- Exposure of breast cancer cells to a subcytotoxic dose of apigenin causes growth inhibition, oxidative stress, and hypophosphorylation of Akt
- Apigenin, a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells
- Apigenin protects ovalbumin-induced asthma through the regulation of Th17 cells
- The IL-23/IL-17 axis in inflammation
- Apigenin Suppresses the IL-1β-Induced Expression of the Urokinase-Type Plasminogen Activator Receptor by Inhibiting MAPK-Mediated AP-1 and NF-κB Signaling in Human Bladder Cancer T24 Cells
- Inhibitory effect of apigenin on nitric oxide production in chondrocytes induced by IL-1 and LPS
- Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor tyrosine kinases by apigenin circumvent taxol resistance in ovarian cancer cells
- Apigenin Inhibits the Expression of IL-6, IL-8, and ICAM-1 in DEHP-Stimulated Human Umbilical Vein Endothelial Cells and In Vivo
- Apige nin inh ib its indoxyl sul fate-inducedend opla smic reti culum stress andan ti -pro lif era tive pat hways, CHOP and IL -6/p 21, in hu man renal proxim al t ubu lar c
- Antitumor and Anti-Invasive Effect of Apigenin on Human Breast Carcinoma through Suppression of IL-6 Expression
- Sa1794 Apigenin Attenuates Cerulein-Induced Parathyroid Hormone-Related Protein (PTHrP) and IL-6 in a Model of Murine Pancreatitis
- 5,6-Dichloro-ribifuranosylbenzimidazole- and apigenin-induced sensitization of colon cancer cells to TNF-α-mediated apoptosis
- Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages
- Apigenin prevents TNF-α induced apoptosis of primary rat retinal ganglion cells.
- Differential response to apigenin in African American triple-negative breast cancer in reducing TNF-α mediated rise in CXCL1
- Apigenin Exerts Anti-inflammatory Effects in an Experimental Model of Acute Pancreatitis by Down-regulating TNF-α

- Cosmetic composition for skin whitening comprising arctiin, arctigenin or the mixture thereof as active
- Anti-aging agent containing arctigenin derivative
- Arctigenin exerts anti-colitis efficacy through inhibiting the differentiation of Th1 and Th17 cells via an mTORC1-dependent pathway
- Arctigenin Suppress Th17 Cells and Ameliorates Experimental Autoimmune Encephalomyelitis Through AMPK and PPAR-γ/ROR-γt Signaling
- Arctigenin functions as a selective agonist of estrogen receptor β to restrict mTORC1 activation and consequent Th17differentiation
- Arctigenin attenuates imiquimod-induced psoriasis-like skin lesions via down-regulating keratin17
- Arctigenin regulates Th1 and Th17differentiation and ameliorates experimental autoimmune encephalomyelitis (THER7P.958)
- Arctigenin improves vascular tone and decreases inflammation in human saphenous vein
- Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway
- Arctigenin , a phenylpropanoid dibenzylbutyrolactone lignan, inhibits type I–IV allergic inflammation and pro-inflammatory enzymes
- Arctigenin Treatment Protects against Brain Damage through an Anti-Inflammatory and Anti-Apoptotic Mechanism after Needle Insertion
- Arctigenin Protects against Lipopolysaccharide-Induced Pulmonary Oxidative Stress and Inflammation in a Mouse Model via Suppression of MAPK, HO-1, and iNOS Signaling
- Anti-inflammatory activity of arctigenin from Forsythiae Fructus
- Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing M1 macrophages to M2-like macrophages
- Arctigenin but not arctiin acts as the major effective constituent of Arctium lappa L. fruit for attenuating colonicinflammatory response induced by dextran sulfate sodium in mice
- Arctigenin from Arctium lappa inhibits interleukin-2 and interferon gene expression in primary human T lymphocytes
- Arctigenin improves vascular tone and decreases inflammation in human saphenous vein
- Anti-inflammatory activity of arctigenin from Forsythiae Fructus
- In vitro anti-inflammatory effects of arctigenin , a lignan from Arctium lappa L., through inhibition on iNOS pathway
- Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing M1 macrophages to M2-like macrophages
- Arctigenin , a phenylpropanoid dibenzylbutyrolactone lignan, inhibits type I–IV allergic inflammation and pro-inflammatory enzymes
- Arctigenin Protects against Lipopolysaccharide-Induced Pulmonary Oxidative Stress and Inflammation in a Mouse Model via Suppression of MAPK, HO-1, and iNOS Signaling
- Arctigenin protects focal cerebral ischemia-reperfusion rats through inhibiting neuroinflammation
- Arctigenin , a Natural Lignan Compound, Induces Apoptotic Death of Hepatocellular Carcinoma Cells via Suppression of PI3‐K/Akt Signaling
- β-Catenin Mediates Anti-adipogenic and Anticancer Effects of arctigenin in Preadipocytes and Breast Cancer Cells
- Protective Effects of arctigenin and Arctiin in H_2O_2-Treated SHSY_5Y Cells
- Arctigenin suppresses inflammation and plays a neuroprotective effect in mice with spinal cord injury
- Arctigenin exerts protective effects against myocardial infarction via regulation of iNOS, COX‑2, ERK1/2 and HO‑1in rats
- Arctigenin Ameliorates Inflammation by Regulating Accumulation and Functional Activity of MDSCs in Endotoxin Shock
- Overview of the anti-inflammatory effects, pharmacokinetic properties and clinical efficacies of arctigenin and arctiin from Arctium lappa L

Asiasari Radix
- The hair growth promoting effect of Asiasari radix extract and its molecular regulation
- KESHARAJA: HAIR VITALIZING HERBS
- Aqueous extract of Asiasari radix inhibits formalin-induced hyperalgesia via NMDA receptors
- Comparative Hair Restorer Efficacy of Medicinal Herb on Nude (Foxn) Mice
- Hair Growth: Focus on Herbal Therapeutic Agent
- Plants used for hair growth promotion:A review
- Regulatory Effect of Inflammatory Reaction of Asiasari Radix
- Phytochemical, Toxicological and Pharmacological Studies of Asiasari Radix et Rhizoma: A Review
- Hair Loss and the Applied Techniques for Identification of Novel Hair Growth Promoters for Hair Re-Growth
- Medical Treatment of Hair Loss
- Hair Growth-Promoting Effects in C57BL/6 Mice
- Anti-oxidation and Anti-inflammatory Effect of Asiasari Radix in RAW 264.7 Cells
- Partially purified Asiasari radix inhibits melanogenesisthrough extracellular signal-regulated kinase signaling in B16F10 cells
- Effect of Aqueous Extract from Asiasari Radixon α
-melanocyte Stimulating Hormone Induced Melanogenesis in B16F10 Melanoma Cells - Study on Advances of Medicine and Its Active Ingredient on the Hair Follicle of Different Animals
- Studies on Antitussive Principles of Asiasari Radix
- Effects of Asiasari radix on the morphology and viability of mesenchymal stem cells derived from the gingiva
- Protection of brain cells against AMPA‐induced damage by Asiasari radix extracts
- Study of components in crude drugs by head space gas chromatography. I. Components of Asiasari radix
- Composition containing Asiasari Radix extractsfor protecting brain cells and improving memory
- Studies of Inhibitory Mechanism on Melanogenesis by Partially Purified Asiasari radixin α-MSH Stimulated B16F10 Melanoma Cells
- Asiasari radix was demonstrated to stimulate hair growth in C57BL/6 and C3H mice by increasing the proliferation of HaCaT and human dermal papilla cells (DPCs) and inducing the expression of VEGF in human DPCs
- Studies of Inhibitory Mechanism on Melanogenesis by Partially Purified Asiasari radix in α-MSH
- Isolation of Five Compounds from Asiasari Radix
- Effects of aqueous extracts from Asiasari Radix on α-melnocyte stimulating hormone induced melanogenesis in B16F10 mouse melanoma cell
- Alternative Medicine for Hair Loss
- Anticancer potential of an ethanol extract of Asiasari radixagainst HCT-116 human colon cancer cells in vitro
- Isolation of a cytotoxic agent from Asiasari Radix
- Insulin inhibits AMPA-induced neuronal damage via stimulation of protein kinase B (Akt)
- Immunopharmacological studies on the anti-allergic actions of traditional Chinese medicines and related components

astilbin
- t-Flavanone Improves the Male Pattern of Hair Loss by Enhancing Hair-Anchoring Strength: A Randomized, Double-Blind, Placebo-Controlled Study
- Mechanism and effect of Shijueming (Concha Haliotidis) on serum calcium in spontaneously hypertensive rats

Astragali Radix
- Radix Astragali injection enhances recovery from acute acoustic trauma
- Inhibitory activities against testosterone 5α-reductase and its hair growth promotion activities
- Review of Astragali Radix
- New Isoflavonoid Glycosides and Related Constituents from Astragali Radix (Astragalus membranaceus) and Their Inhibitory Activity on Nitric Oxide Production
- Comparative analysis of multiple representative components in the herb pair Astragali Radix-Curcumae Rhizoma and its single herbs by UPLC-QQQ-MS
- Astragali radix: could it be an adjuvant for oxaliplatin-induced neuropathy?
- Effects of Traditional Chinese Medicine Huangqi Injection (Radix astragali)on Random Skin Flap Survival in Rats
- Radix astragali injection enhances recovery from sudden deafness
- Herbal composition for the treatment of alopecia
- Hair growershaving actions of promoting proliferation of hair papilla cells
- The Effects of Shi-Quan-Dai-Bu-Tang and Its Ingredients on the Survival of Jejunal Crypt Cells and Hematopoietic Cells in Irradiated Mice
- Hair growth effectof traditional Chinese medicine BeauTop on androgenetic alopecia patients: A randomized double-blind placebo-controlled clinical trial
- Effect of Herbal Medicines Pharmacopuncture on Hair Growth,a Review of Animal Study Reports Published in Koreae
- Medical herb composition for promoting the growth of hairA mammal and the manufacturing method thereof
- Astragali Radix elicits anti-inflammation via activation of MKP-1, concomitant with attenuation of p38 and Erk
- Pro-inflammatory cytokine gene expression and nitric oxide regulation of aqueous extracted Astragali radix in RAW 264.7 macrophage cells
- Effect of Astragali Radix Extract on Lipopolysaccharide-Induced Inflammation in Human Amnion
- Synergistic interaction between Astragali Radix and Rehmanniae Radix in a Chinese herbal formula to promote diabetic wound healing
- Chinese medicinal herb Radix Astragalisuppresses cardiac contractile dysfunction and inflammation in a ratmodel of autoimmune myocarditis
- Protective effect of Astragali radix extract on interleukin 1β‐induced inflammation in human amnion
- Wound-healing activity of Astragali Radix in rats.
- Alleviation of osteoarthritis by calycosin-7-O-β-d-glucopyranoside (CG) isolated from Astragali radix (AR) in rabbit osteoarthritis (OA) model
- Isolation of Hyaluronidase Inhibitory Component from the Roots of Astraglus membranaceus Bunge (Astragali Radix)
- Anti-atherosclerotic function of Astragali Radix extract:downregulation of adhesion molecules in vitro and in vivo
- Inhibitory Effect of Astragali Radix on Matrix Degradation in Human Articular Cartilage
- Phenolic Derivatives from Radix Astragaliand their Anti-inflammatory Activities
- Immune-enhancing effect of Danggwibohyeoltang, an extract from Astragali Radixand Angelicae gigantis Radix, in vitro and in vivo
- Immunomodulatory Effect of Astragali Radix Extract on Murine Th1/Th2 Cell Lineage Development
- Transcriptional profiling of human skin fibroblast cell lineHs27 induced by herbal formula Astragali Radixand Rehmanniae Radix
- Ethanolic Extract of Astragali Radix and Salviae Radix Prohibits Oxidative Brain Injury by Psycho-Emotional Stress in Whisker Removal Rat Model
- Effect on TNF-α,IL-1 and IL-6 of Viral Myocarditis Treated by Radix Astragali Injection
- Effects of Radix astragali inoculation fluid on serum TNF-α and ET-1 levels in patients with acute cerebral infarction and significance.
- The effects of Radix astragali on TNF-α, IL-6 in serum of infants with rotavirus enteritis
- Effect of Acupuncture and Radix Astragali aqua-acupuncture at Synsu(BL23) on transcriptional expression of mouse cytokine IL-6

- Baicalin inhibits IL-17-mediated joint inflammation in murine adjuvant-induced arthritis
- Identification of Baicalin as an Immunoregulatory Compound by Controlling TH17 Cell Differentiation
- Baicalin Alleviates Silica-Induced Lung Inflammation and Fibrosis by Inhibiting the Th17 Response in C57BL/6 Mice
- Baicalin inhibits IgG production by regulating Treg/Th17 axis in a mouse model of red blood cell transfusion
- Identification of Baicalin as an Immunoregulatory Compound by Controlling TH17 Cell Differentiation
- Baicalin Attenuates IL-17-Mediated Acetaminophen-Induced Liver Injury in a Mouse Model
- Baicalin attenuates TNBS-induced colitis in rats by modulating the Th17/Treg paradigm
- Baicalin Alleviates Silica-Induced Lung Inflammation and Fibrosis by Inhibiting the Th17Response in C57BL/6 Mice
- Regulatory effect of Baicalin on the imbalance of Th17/Treg responses in mice with allergic asthma
- Study on the inhibitory activity, in vitro, of baicalein and Baicalin against skin fungi and bacteria
- Baicalin protects human skin fibroblasts from ultraviolet A radiation-induced oxidative damage and apoptosis
- Baicalin modulates microRNA expression in UVB irradiated mouse skin
- Effect of low molecular weight chitosans on drug permeation through mouse skin: 1. Transdermal delivery of Baicalin
- Evaluation of efficacy and tolerance of a nighttime topical antioxidant containing resveratrol, Baicalin , and vitamin e for treatment of mild to moderately photodamaged skin.
- The Effects of Baicalin Against UVA-Induced Photoaging in Skin Fibroblasts
- Protective effect of Baicalin against multiple ultraviolet b exposure-mediated injuries in C57BL/6 mouse skin
- Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways
- Effects of dietary Baicalin supplementation on iron overload-induced mouse liver oxidative injury
- The flavonoid Baicalin counteracts ischemic and oxidative insults to retinal cells and lipid peroxidation to brain membranes
- Baicalin prevents the production of hydrogen peroxide and oxidative stress induced by Aβ aggregation in SH-SY5Y cells
- Baicalin protects human skin fibroblasts from ultraviolet A radiation-induced oxidative damage and apoptosis
- Baicalin Attenuates Alcoholic Liver Injury through Modulation of Hepatic Oxidative Stress, Inflammation and Sonic Hedgehog Pathway in Rats
- Short-term feeding of Baicalin inhibits age-associated NF-κB activation
- Baicalin ameliorates isoproterenol-induced acute myocardial infarction through iNOS, inflammation, oxidative stress and P38MAPK pathway in rat
- Baicalin attenuates oxygen-glucose deprivation-induced injury by inhibiting oxidative stress-mediated 5-lipoxygenase activation in PC12 cells
- Baicalin protects PC-12 cells from oxidative stress induced by hydrogen peroxide via anti-apoptotic effects
- Baicalin prevents cadmium induced hepatic cytotoxicity, oxidative stress and histomorphometric alterations
- Baicalin ameliorates isoproterenol-induced acute myocardial infarction through iNOS, inflammation and oxidative stress in rat
- Protective Effects of Baicalin on Aβ1–42-Induced Learning and Memory Deficit, Oxidative Stress, and Apoptosis in Rat
- The Protective Effect of Baicalin Against Lead-Induced Renal Oxidative Damage in Mice
- Study of the Effect of Baicalin and Its Natural Analogs on Neurons with Oxygen and Glucose Deprivation Involving Innate Immune Reaction of TLR2/TNF ?
- Baicalin Downregulates Porphyromonas gingivalis Lipopolysaccharide-Upregulated IL-6 and IL-8 Expression in Human Oral Keratinocytes by Negative Regulation of TLR Signaling
- Baicalin and Baicalein Inhibit Src Tyrosine Kinase and Production of IL-6
- Baicalin Ameliorates Dysimmunoregulation in Pristane-Induced Lupus Mice: Production of IL-6 and PGE2 and Activation of T cells
- Effects of baicalin on TNF-α, IL-6 and IL-10 in rats with severe acute pancreatitis.
- A Study of Baicalin Inhibiting the Activation of NF-kB and Synthesise of IL-6 in Systemic Inflammatory Response Syndrome
- Inhibitory effects of baicalin on IL-1β- induced MMP-1/TIMP-1 and its stimulated effect on Collagen-I production in human periodontal ligament cells
- The anti‐inflammatory effect of baicalin on hypoxia/reoxygenation and TNF‐α induced injury in cultural rat cardiomyocytes
- Effects of Baicalin and Octreotide on the Serum TNF-α Level and Apoptosis in Multiple Organs of Rats with Severe Acute Pancreatitis
- Influence of baicalin on TNF-α mRNA, caspase-3 and P-selectin expression in pancreatic tissue of rats with severe acute pancreatitis
- Analysis of Influence of Baicalin Joint Resveratrol Retention Enema on the TNF-α, SIgA, IL-2, IFN-γ of Rats with Respiratory Syncytial Virus Infection
- Baicalin protects against TNF-α-induced injury by down-regulating miR-191a that targets the tight junction protein ZO-1 in IEC-6 cells
- Effects of Baicalin on Apoptosis of Early Chorionic Trophoblast Cell Cultures Induced by Exosomatic TNF-α
- Effects of Baicalin on the expression of TNF-α, IL-1β in cerebraltissue after local cerebral ischemia-reperfusion in rats
- Baicalin suppresses NLRP3 inflammasome and nuclear factor-kappa B (NF-κB) signaling during Haemophilus parasuis infection
- Baicalin inhibitednuclear factor κB (NF-κB) activation and attenuatedsodium taurocholate of induced experimental pancreatitis in rats

- Suppression of interleukin 17production by Brazilian propolis in mice with collagen-induced arthritis
- Brazilian propolis inhibits the differentiation of Th17 cells by inhibition of interleukin-6-induced phosphorylation of signal transducer and activator of transcription 3
- Neovestitol, an isoflavonoid isolated from Brazilian red propolis, reduces acute and chronic inflammation: involvement of nitric oxide andIL-6
- Brazilian propolis ameliorates trinitrobenzene sulfonic acid-induced colitis in mice by inhibiting Th1 differentiation
- Brazilian Green Propolis: Anti-Inflammatory Property by an Immunomodulatory Activity
- Antinociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents
- Antimicrobial Brazilian Propolis (EPP-AF) Containing Biocellulose Membranes as Promising Biomaterial for Skin Wound Healing
- Propolis effect on Th1/Th2 cytokines production by acutely stressed mice
- Nitric Oxide and Brazilian Propolis Combined Accelerates Tissue Repair by Modulating Cell Migration, Cytokine Production and Collagen Deposition in Experimental Leishmaniasis
- Stimulatory Effect of Brazilian Propolis on Hair Growth through Proliferation of Keratinocytes in Mice
- Brazilian green propolis improves immune function in aged mice
- Brazilian propolis protects Saccharomyces cerevisiae cells against oxidative stress
- Evaluation of the Potential of Brazilian Propolis against UV-Induced Oxidative Stress
- Green Brazilian propolis effects on sperm count and epididymis morphology and oxidative stress
- Baccharis dracunculifolia, the main source of green propolis, exhibits potent antioxidant activity and prevents oxidative mitochondrial damage
- Anti-Inflammatory and Antimicrobial Evaluation of Neovestitol and Vestitol Isolated from Brazilian Red Propolis
- Propolis: a review of its anti-inflammatory and healing actions
- Topical Brazilian propolis improves corneal wound healing and inflammation in rats following alkali burns
- Aqueous Extract of Brazilian Green Propolis: Primary Components, Evaluation of Inflammation and Wound Healing by Using Subcutaneous Implanted Sponges
- Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages
- Propolis Induces Analgesic and Anti-inflammatory Effects in Mice and Inhibits In Vitro Contraction of Airway Smooth Muscle
- Brazilian green propolis modulates inflammation, angiogenesis and fibrogenesis in intraperitoneal implant in mice
- Propolis immunomodulatory action in vivo on Toll‐like receptors 2 and 4 expression and on pro‐inflammatory cytokines production in mice
- Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatoryresponse by blocking NF-κB and MAPK activation in macrophages
- Brazilian Red Propolis AttenuatesInflammatory Signaling Cascade in LPS-Activated Macrophages
- Molecular Mechanisms Underlying the In Vitro Anti-Inflammatory Effects of a Flavonoid-Rich Ethanol Extract from Chinese Propolis (Poplar Type)
- Chemical Characterization and Antioxidant, Antimicrobial, and Anti-Inflammatory Activities of South Brazilian Organic Propolis
- Chemical Constituents of Brazilian Propolis and Their Cytotoxic Activities
- Two Novel Cytotoxic Benzofuran Derivatives from Brazilian Propolis
- Correlation analysis between phenolic levels of Brazilian propolis extracts and their antimicrobial and antioxidant activities
- Brazilian propolis-derived components inhibit TNF-α-mediated downregulation of adiponectin expression via different mechanisms in 3T3-L1 adipocytes

Buxus Wallichiana Baill
- Histological and physico-chemical evaluation of Buxus wallichiana Baill.
- Evaluation of Hair Growth Activity of Buxus wallichianaBaill Extract in Rats
- Buxus wallichiana L., a multipurpose Himalayan tree in peril
- Preclinical studies of a novel polyherbal phyto–complex hair growth promoting cream
- Histological and physico-chemical evaluation of Buxus wallichiana Baill
- System and method for promoting hair growth and improving hair and scalp health
- Evaluation of antioxidant and antimicrobial activity of various extracts of Buxus wallichiana Baill wood
- PRELIMINARY ANTINFLAMMATORY STUDYOF DIFFERENT EXTRACTS OF BUXUS WALLICHIANA BAILL WOOD
- Powder microscopy and phytochemical screening on stem bark and leaves of Buxus wallichiana Baill – Buxaceae
- PROSPECT OF HERBS AS HAIR GROWTH POTENTIAL

- Capparis Spinosa L. promotes anti-inflammatory response in vitro through the control of cytokine gene expression in human peripheral blood mononuclear cells
- Anti-inflammatory potential of Capparis spinosa L. in vivo in mice through inhibition of cell infiltration and cytokine gene expression
- Anti-inflammatory Effects of Caper (Capparis spinosa L.) Fruit Aqueous Extract and the Isolation of Main Phytochemicals
- Capparis ovata treatment suppresses inflammatorycytokine expression and ameliorates experimental allergic encephalomyelitis model of multiple sclerosis in C57BL/6 mice
- Evaluation of anti-bacterial activity of Capparis spinosa (Al-Kabara ) and Aloe vera extracts against Isolates Bacterial Skin Wound Infections in -vitro and in-vivo
- Isolation and identification of an anti-inflammatoryprinciple from Capparis spinosa.
- Biflavonoids from Caper (Capparis spinosa L.) Fruits and Their Effects in Inhibiting NF-kappa B Activation
- Effect of flavonoids rich extract of Capparis spinosa oninflammatory involved genes in amyloid-beta peptide injected rat model of Alzheimer’s disease
- Investigation for anti-inflammatory and anti-thrombotic activities of methanol extract of Capparis ovata buds and fruits
- Capparis spinosa protects against oxidative stress in systemic sclerosis dermal fibroblasts
- In vitro antioxidant and in vivo photoprotective effects of a lyophilized extract of Capparis spinosa L. buds
- Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovata Desf. to drought
- Phenolic Compounds and Vitamin Antioxidants of Caper (Capparis spinosa)
- Effect of Tunisian Capparis spinosa L. extract on melanogenesis in B16 murine melanoma cells
- ROLES OF DURATION AND CONCENTRATION OF PRIMING AGENTS ON DORMANCY BREAKING AND GERMINATION OF CAPER (CAPPARIS SPINOSA L.) FOR THE PROTECTION OF ARID DEGRADED AREAS
- Biflavonoids from Caper (Capparis spinosa L.) Fruits and Their Effects in Inhibiting NF-kappa B Activation

- Carnosol Modulates Th17 Cell Differentiation and Microglial Switch in Experimental Autoimmune Encephalomyelitis
- Inhibitory effect of Carnosol on UVB-induced inflammation via inhibition of STAT3
- Inhibitory Effect of Carnosolon Phthalic Anhydride-Induced Atopic Dermatitis via Inhibition of STAT3
- Carnosol Inhibits Pro-Inflammatory and Catabolic Mediators of Cartilage Breakdown in Human Osteoarthritic Chondrocytes and Mediates Cross-Talk between Subchondral Bone Osteoblasts and Chondrocytes
- Anti‐inflammatoryactivity of rosemary extracts obtained by supercritical carbon dioxide enriched in carnosic acid and carnosol
- Carnosol protects against renal ischemia-reperfusion injury in rats
- Carnosol: A promising anti-cancer and anti-inflammatory agent
- Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation
- Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-κB in mouse macrophages
- The Mechanisms of Carnosol in Chemoprevention of Ultraviolet B-Light-Induced Non-Melanoma Skin Cancer Formation
- Syk/Src Pathway-Targeted Inhibition of Skin InflammatoryResponses by Carnosic Acid
- Carnosol and Related Substances Modulate Chemokine and Cytokine Production in Macrophages and Chondrocytes
- Carnosol inhibits cell adhesion molecules and chemokine expression by tumor necrosis factor-α in human umbilical vein endothelial cells through the nuclear factor-κB and mitogen-activated protein kinase pathways
- Carnosic acid reduces cytokine-induced adhesion molecules expression and monocyte adhesion to endothelial cells
- In vitro and in vivo anti-inflammatory effects of rosmanol and carnosol isolated from rosemary
- Carnosol promotes endothelial differentiation under H2O2-induced oxidative stress
- Anti‐inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions
- The Role of Nitric Oxide Synthase and Carnosol in UVB-induced NF-κB Activity and Skin Damage
- Protective effects of carnosol against oxidative stress induced brain damage by chronic stress in rats
- Upregulation of NF-E2-related factor-2-dependent glutathione by carnosol provokes a cytoprotective response and enhances cell survival

Ceratonia Siliqua
- Phenolic acid content and free radical-scavenging activity of two differently processed carob tree (Ceratonia siliqua L.) pod
- Structure and Development of Stomata on the Primary Root of Ceratonia siliqua L.
- Reduction of hair growth
- Protective effects of polyphenol-rich infusions from carob (Ceratonia siliqua) leaves and cladodes of Opuntia ficus-indica against inflammation associated with diet-induced obesity and DSS-induced colitis in Swiss mice
- Screening of indigenous Palestinian medicinal plants for potential anti-inflammatory and cytotoxic activity
- Extract from Ceratonia siliqua Exhibits Depigmentation Properties
- Anti-inflammatory and antioxidant effect of Ceratonia siliqua L. methanol barks extract
- Carob pods (Ceratonia siliqua L.)inhibit human neutrophils myeloperoxidase and in vitro ROS-scavenging activity
- Gastroprotective effect of carob (Ceratonia siliqua L.)against ethanol-induced oxidative stress in rat
- The Study of Antibacterial Activity of Plantago Major and Ceratonia Siliqua
- Anti-Inflammatory Activity of Natural Products
- Protective Effect of Ceratonia siliqua L. Against a Dextran Sulfate Sodium-Induced Alterations in Liver and Kidney in Rat
- In vitro antioxidant and inhibitory activity of water decoctions of carob tree (Ceratonia siliqua L.) on cholinesterases, α-amylase and α-glucosidase
- Effects of extraction conditions on the recovery of phenolic compounds and in vitro antioxidant activity of carob (Ceratonia siliqua L.) pulp
- Characterization of bioactive compounds and ameliorative effects of Ceratonia siliqua leaf extract against CCl4 induced hepatic oxidative damageand renal failure in rats
- Ceratonia siliqua honeys from Morocco: Physicochemical properties, mineral contents, and antioxidant activities
- Ceratonia siliqua L. (immature carob bean) inhibits intestinal glucose absorption, improves glucose tolerance and protects against alloxan‐induced diabetes in rat
- Effects of aqueous extracts from Ceratonia siliqua L. pods on small intestinal motility in rats and jejunal permeability in mice
- Ceratonia siliqua pod extract ameliorates Schistosomamansoni-induced liver fibrosis and oxidative stress
- Bioactive metabolites involved in the antioxidant, anticancer and anticalpain activities of Ficus carica L., Ceratonia siliqua L. and Quercus ilex L. extracts

Chrysanthemum Zawadskii
- In vivo hair growth-stimulating effect of medicinal plant extract on BALB/c nude mice
- Chrysanthemum zawadskii extract induces hair growthby stimulating the proliferation and differentiation of hair matrix
- Phylogenetic analysis of Korean native Chrysanthemum species based on morphological characteristics
- Medicinal Plants for the Treatment of Hair Lossand the Suggested Mechanisms
- Hair growth promoting effect and action mechanism of Chrysanthemum zawadskii extract
- Hair restorer compositionusing oriental herbs
- Inhibitory Effects of Chromatographically Fractionated Extracts from Chrysanthemum zawadskii on Tyrosinase Activity and Melanogenesis
- The Functional Effects for the Prevention and Treatment on Hair Loss from Astringent Persimmon Fruit Extracts
- Growth and Morphological Characteristics of Wild Clones of Chrysanthemumboreale Mak
- Biological activities of the herb of Chrysanthemum zawadskii
- Inhibitors of Nitric Oxide in Raw 264.7 Macrophages treated with Linarin: The main compound of Chrysanthemum zawadskii
- Chrysanthemum zawadskii extract activates peroxisome proliferator-activated receptor-α and has an anti-inflammatory activity : Potential interest for the skin barrier function
- Immuno-Modulatory Activities of Polysaccharides separated from Chrysanthemum zawadskii var. latilobum in Macrophage Cells
- Effect of Chrysanthemum zawadskii and Menthaarvensis on skin barrier functionvia keratinocytes differentiation
- Abstract 5666: Nrf2-mediated induction of phase 2 detoxifying enzymes by an active compound isolated from Chrysanthemum zawadskii
- Chrysanthemum zawadskiivar. latilobum extract inhibits the production of nitric oxide and PGE2 through inducible nitric oxide synthase (iNOS) and cyclooxygenase-2(COX-2) in RAW 264. 7 cells
- Anti-inflammatory/Anti-oxidative Stress Activitiesand Differential Regulation of Nrf2-Mediated Genes by Non-Polar Fractions of Tea Chrysanthemum zawadskiiand Licorice Glycyrrhiza uralensis
- Anti-inflammatory activity of Chrysanthemum zawadskiivar. latilobum leaf extract through haem oxygenase-1 induction
- 6-Methoxyluteolin from Chrysanthemum zawadskii var.latilobum SuppressesHistamine Release and Calcium Influx via Down-Regulation of FcεRI αChain Expression
- Anti-inflammatory effectsof flavonoids in Korean Chrysanthemum speciesvia suppression of inducible nitric oxide synthase and cyclooxygenase-2 in LPS-induced RAW 264.7 macrophages
- Suppressive Effects of Chrysanthemum zawadskii var.latilobum Flower Extracts on Nitric Oxide Production and Inducible Nitric Oxide Synthase Expression
- Inhibitory Effects of 150 Plant Extracts on Elastase Activity, and Their Anti‐inflammatory Effects
- Isolation and identification of flavonoids from Gujeolcho (Chrysanthemum zawadskii var. latilobum) as inhibitor of histamine release
- A Study on Anti-oxidative, Anti-inflammatory,and Melanin Inhibitory Effects of Chrysanthemum Sibiricum Extract
- Articles : Analysis of Composition and Activity of Essential Oil from Chrysanthemum zawadskii var. Latilobum and C. indicum against Antibiotic-Resistant Pathogenic Bacteria
- Linarin down-regulates phagocytosis, pro-inflammatory cytokine production, and activation marker expression in RAW264.7 macrophages
- The Extract of Chrysanthemum zawadskiivar. latilobum Ameliorates Collagen-Induced Arthritis in Mice
- Chrysanthemum zawadskiiextract protects osteoblastic cells from highly reducing sugar-induced oxidative damage
- Identification of secondary metabolites with antioxidant and antimicrobial activities from Artemisia iwayomogi and Chrysanthemum zawadskii
- Extracts of Chrysanthemum zawadskii attenuate oxidative damage to vascular endothelial cells caused by a highly reducing sugar
- Chrysanthemum zawadskii var. latilobum Extracts Inhibits of TPA-induced Invasion by Reducing MMP-9 Expression Via the Suppression of NF-κB
Activation in MCF-7 Human Breast Carcinoma Cells

- The Effects of Cordycepin on Ovalbumin-Induced Allergic Inflammation by Strengthening Treg Response and Suppressing Th17 Responses in Ovalbumin-Sensitized Mice
- Cordycepin and a preparation from Cordyceps militaris inhibit malignant transformation and proliferation by decreasing EGFR and IL-17RA signaling in a murine oral cancer model
- Cordycepin Inhibits Differentiation of Th17 Cells and Promotes Generation of CD4+ CD25+ FoxP3+ Tregs In Vitro : 1100
- Cordycepin induces apoptosis in SGC‑7901 cells through mitochondrial extrinsic phosphorylation of PI3K/Akt by generating ROS
- Cordyceps sinensis may inhibit Th22 cell chemotaxis to improve kidney function in lgA nephropathy
- Effect of Cordycepinon the Expression of the Inflammatory CytokinesTNF-alpha, IL-6, and IL-17A in C57BL/6 Mice
- Cordycepin inhibits IL-1β-induced MMP-1 and MMP-3 expression in rheumatoid arthritis synovial fibroblasts
- Cordycepin prevented IL-β-induced expression of inflammatory mediators in human osteoarthritis chondrocytes
- Nucleotides. Part IL.† Synthesis and Characterization of Cordycepin‐Trimer‐Vitamin and ‐Lipid Conjugates Potential Inhibitors of HIV‐1 Replication
- Effect of CordycepinPurified from Cordyceps militaris on Th1 and Th2Cytokines in Mouse Splenocytes
- Cordycepin Suppresses Expression of Diabetes Regulating Genes by Inhibition of Lipopolysaccharide-induced Inflammation in Macrophages
- A Phytochemically Characterized Extract of Cordyceps militaris and Cordycepin Protect Hippocampal Neurons from Ischemic Injury in Gerbils
- Role of Cordycepin and Adenosine on the Phenotypic Switch of Macrophages via Induced Anti-inflammatory Cytokines
- Cordycepin modulates inflammatory and catabolic gene expression in interleukin-1beta-induced human chondrocytes from advanced-stage osteoarthritis: an in vitro study
- Cordycepin-enriched Cordyceps militaris induces immunomodulation and tumor growth delay in mouse-derived breast cancer
- Regulation of human cytokines by Cordyceps militaris
- Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets
- Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress
- Effect of Long-term Administration of Cordycepin from Cordyceps militaris on Testicular Function in Middle-aged Rats
- Cordycepin alleviates airway hyperreactivity in a murine model of asthma by attenuating the inflammatory process
- Anti-hepatocarcinoma effect of cordycepin against NDEA-induced hepatocellular carcinomas via the PI3K/Akt/mTOR and Nrf2/HO-1/NF-κB pathway in mice
- Cordycepin diminishes thymic stromal lymphopoietin-induced interleukin-13 production
- Cordycepin Suppresses Thymic Stromal Lymphopoietin Expression via Blocking Caspase-1 and Receptor-Interacting Protein 2 Signaling Pathways in Mast Cells
- Cordycepin alleviates lipopolysaccharide-induced acute lung injury via Nrf2/HO-1 pathway
- Protective effects of cordycepin on the histopathological changes and oxidative stress induced by hepatic ischemia/reperfusion in rats
- Cordycepin inhibits LPS-induced inflammatory responses by modulating NOD-Like Receptor Protein 3 inflammasome activation
- Cordycepin (3′-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats
- The neuroprotective effects of cordycepin inhibit glutamate-induced oxidative and ER stress-associated apoptosis in hippocampal HT22 cells
- Cordycepinprevents oxidative stress-induced inhibition of osteogenesis
- Polysaccharides from Cordyceps sinensis mycelium ameliorate exhaustive swimming exercise-induced oxidative stress
- Cordycepin suppresses TNF-α-induced NF-κB activation by reducing p65 transcriptional activity, inhibiting IκBα phosphorylation, and blocking IKKγ ubiquitination
- Cordycepin as a sensitizer to tumour necrosis factor (TNF)‐α‐induced apoptosis through eukaryotic translation initiation factor 2α (eIF2α)‐ and mammalian target of rapamycin complex 1 (mTORC1)‐mediated inhibition of nuclear factor (NF)‐κB
- Cordycepin protected against the TNF-α-induced inhibition of osteogenic differentiation of human adipose-derived mesenchymal stem cells
- Cordycepin inhibits vascular adhesion molecule expression in TNF‑α‑stimulated vascular muscle cells
- Cordycepin as a sensitizer to tumour necrosis factor (TNF)-α-induced apoptosis through eukaryotic translation initiation factor 2α (eIF2α)-and mammalian target of rapamycin complex 1 (mTORC1)-mediated inhibition of nuclear factor (NF)-κB

- Inhibition of interleukin‐17‐stimulated interleukin‐6 and ‐8 production by cranberry components in human gingival fibroblasts and epithelial cells
- Effects of cranberry components on IL-1β-stimulated production of IL-6, IL-8 and VEGF by human TMJ synovial fibroblasts
- Anti-Porphyromonas gingivalis and Anti-Inflammatory Activities of A-Type Cranberry Proanthocyanidins
- Cranberry Proanthocyanidins Improve Intestinal sIgA During Elemental Enteral Nutrition
- Cranberry components inhibit interleukin‐6, interleukin‐8, and prostaglandin E2 production by lipopolysaccharide‐activated gingival fibroblasts
- Effect of glycated albumin and cranberry components on interleukin‐6 and matrix metalloproteinase‐3 production by human gingival fibroblasts
- Cranberry extract suppresses interleukin-8 secretion from stomach cells stimulated by Helicobacter pylori in every clinically separated strain but inhibits growth in part of the strains
- Inhibition of interleukin 1β–stimulated interleukin‐6 production by cranberry components in human gingival epithelial cells: effects on nuclear factor κB and activator protein 1 activation pathways
- Comparison of Urinary Cytokines after Ingestion of Cranberry Juice Cocktail in Pregnant Subjects: A Pilot Study
- Anti-inflammatory Activity of a High-molecular-weight Cranberry Fraction on Macrophages Stimulated by Lipopolysaccharides from Periodontopathogens
- Effects of freeze-dried cranberry powder on serum lipids and inflammatory markers in lipopolysaccharide treated rats fed an atherogenic diet
- Effects of cranberry extracts and ursolic acid derivatives on P-fimbriated Escherichia coli, COX-2 activity, pro-inflammatory cytokine release and the NF-κβ transcriptional response in vitro
- Green tea polyphenol epigallocatechin-3-gallate and cranberry proanthocyanidins act in synergy with cathelicidin (LL-37) to reduce the LPS-induced inflammatory response in a three-dimensional co-culture model of gingival epithelial cells and fibroblasts
- Effects of ginger and cranberry extracts on the physiological response to exercise and markers of inflammation in horses
- Prevention of oxidative stress, inflammationand mitochondrial dysfunction in the intestine by different cranberry phenolic fractions
- Diets enriched with cranberry beans alter the microbiota and mitigate colitis severity and associated inflammation
- Anti-inflammatory effects of cranberry juice in lipopolysaccharide (LPS)-stimulated RAW 264.7 murine macrophage cells.
- Cranberry extract attenuates hepatic inflammation in high-fat-fed obese mice
- Effects of cranberry (Vaccinum macrocarpon) supplementation on iron status and inflammatory markers in rowers
- Cytoprotective effect of Proanthocyanidin‐rich cranberry fraction against bacterial cell wall‐mediated toxicity in macrophages and epithelial cells
- Cranberry Extract Modulate Inflammation In Irradiated Rats.
- Chapter 81 – Cranberry Polyphenols: Effects on Cardiovascular Risk Factors
- Preventive effect of a pectic polysaccharide of the common cranberry Vaccinium oxycoccos L. on acetic acid-induced colitis in mice
- Cranberry extract inhibits low density lipoprotein oxidation
- Reduced-energy cranberry juice increases folic acid and adiponectin and reduces homocysteine and oxidative stress in patients with the metabolic syndrome
- Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome
- A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice
- Biological Activity of Cranberry Proanthocyanidins: Effects on Oxidation, Microbial Adhesion, Inflammation, and Health
- Effects of cranberry powder on serum lipid profiles and biomarkers of oxidative stress in rats fed an atherogenic diet
- Effects of cranberry powder on biomarkers of oxidative stress and glucose control in db/db mice
- CRANBERRY POLYPHENOLS DOWN-REGULATE THE TOLL-LIKE RECEPTOR 4 PATHWAY AND NUCLEAR FACTOR-KAPPA B ACTIVATION, WHILE STILL ENHANCING TUMOR NECROSIS FACTOR ΑLPHA SECRETION IN HL-60 CELLS

- Curcumin, an antioxidant and anti-inflammatoryagent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress
- Curcumin, inflammation, ageing and age-related diseases
- Curcumin, Inflammation, and Chronic Diseases: How Are They Linked?
- Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation.
- Effects of curcumin on retinal oxidative stress and inflammation in diabetes
- Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions
- Dietary Curcumin Significantly Improves Obesity-Associated Inflammation and Diabetes in Mouse Models of Diabesity
- Curcumin attenuates allergic airway inflammation by regulation of CD4+CD25+ regulatory T cells (Tregs)/Th17 balance in ovalbumin-sensitized mice
- Curcumin Attenuates Acute Graft-versus-Host Disease Severity via In Vivo Regulations on Th1, Th17 and Regulatory T Cells
- Treatment of low doses curcumin could modulate Th17/Treg balance specifically on CD4+ T cell cultures of systemic lupus erythematosus patients
- Curcumin inhibiting Th17 cell differentiation by regulating the metabotropic glutamate receptor-4 expression on dendritic cells
- Amelioration of experimental autoimmune encephalomyelitis by curcumin treatment through inhibition of IL-17production
- Curcumin protects against acute liver damage in the rat by inhibiting NF-κB, proinflammatory cytokines production and oxidative stress
- Curcumin downregulates the inflammatorycytokines CXCL1 and -2 in breast cancer cells via NFκB
- Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy
- Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokinesCXCL1 and -2
- Inhibition of NFκB Activation with Curcumin Attenuates Plasma InflammatoryCytokines Surge and Cardiomyocytic Apoptosis Following Cardiac Ischemia/Reperfusion1
- Investigation of the Effects of Curcumin on Serum Cytokines in Obese Individuals: A Randomized Controlled Trial
- Curcumin attenuates the release of pro‐inflammatory cytokines in lipopolysaccharide‐stimulated BV2 microglia
- Differential Regulation of Cytokines and Transcription Factors in Liver by Curcumin Following Hemorrhage/Resuscitation
- Amelioration of experimental autoimmune encephalomyelitis by curcumin treatment through inhibition of IL-17 production
- [Effect of curcumin on IL-17-induced nitric oxide production and expression of iNOS in human keratinocytes].
- Imiquimod-induced psoriasis-like inflammation in differentiated Human keratinocytes: Its evaluation using curcumin
- Inhibition of interleukin 17 production by curcumin in mice with collagen-induced arthritis
- Curcumin ameliorates arterial dysfunction and oxidative stress with aging
- Curcumin Extends Life Span, Improves Health Span, and Modulates the Expression of Age-Associated Aging Genes in Drosophila melanogaster
- Curcumin and aging
- Dietary Curcumin Ameliorates Aging-RelatedCerebrovascular Dysfunction through the AMPK/Uncoupling Protein 2 Pathway
- Curcumin induces heme oxygenase‐1 in normal human skin fibroblasts through redox signaling: Relevance for anti‐aging intervention
- Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs
- Curcumin attenuates the expression of IL-1β, IL-6, and TNF-α as well as cyclin E in TNF-α-treated HaCaT cells; NF-κB and MAPKs as potential upstream targets
- Curcumin Attenuates Inflammatory Responses of TNF-α-Stimulated Human Endothelial Cells
- Curcumin Supplementation Lowers TNF-α, IL-6, IL-8, and MCP-1 Secretion in High Glucose-Treated Cultured Monocytes and Blood Levels of TNF-α, IL-6, MCP-1, Glucose, and Glycosylated Hemoglobin in Diabetic Rats
- The antihyperglycemic effect of curcumin in high fat diet fed rats. Role of TNF-α and free fatty acids
- Curcumin Attenuates Diabetic Neuropathic Pain by Downregulating TNF-α in a Rat Model
- Effects of TNF-α and curcumin on the expression of thrombomodulin and endothelial protein C receptor in human endothelial cells
- Curcumin ameliorates rabbits’s steatohepatitis via respiratory chain, oxidative stress, and TNF-α
- Aminoguanidine and curcumin attenuated tumor necrosis factor (TNF)-α-induced oxidative stress, colitis and hepatotoxicity in mice
- Curcumin Attenuates TNF‐α‐induced Expression of Intercellular Adhesion Molecule‐1, Vascular Cell Adhesion Molecule‐1 and Proinflammatory Cytokines in Human Endometriotic Stromal Cells
- Anti-inflammatory potential of curcumin and quercetin in rats: Role of oxidative stress, heme oxygenase-1 and TNF-α
- Curcumin Blocks Interleukin-1 (IL-1) Signaling by Inhibiting the Recruitment of the IL-1 Receptor–Associated Kinase IRAK in Murine Thymoma EL-4 Cells
- Curcumin attenuates carcinogenesis by down regulating proinflammatory cytokine interleukin-1 (IL-1α and IL-1β) via modulation of AP-1 and NF-IL6 in lymphoma bearing mice
- Curcumin alleviates IL‐17A‐mediated p53‐PAI‐1 expression in bleomycin‐induced alveolar basal epithelial cells
- Curcumin attenuates IL-1β-induced endothelial lipase expression via blocking NF-κB pathway
- Curcumin Attenuates Expression of Adhesion Molecules by IL-1 β in HUVECs
- Inhibition of curcumin on the expression of IL-1 β-induced nuclear factor-κB-dependent inflammatory gene in rabbit RPE cells
- Curcumin (Diferuloylmethane) Inhibits Constitutive and IL-6-Inducible STAT3 Phosphorylation in Human Multiple Myeloma Cells
- Curcumin Inhibits Imiquimod-Induced Psoriasis-Like Inflammation by Inhibiting IL-1beta and IL-6 Production in Mice
- Curcumin, a Potential Inhibitor of Up-regulation of TNF-alpha and IL-6 Induced by Palmitate in 3T3-L1 Adipocytes through NF-kappaB and JNK Pathway
- Anti-ulcer Activity of Curcumin on Experimental Gastric Ulcer in Rats and Its Effect on Oxidative Stress/Antioxidant, IL-6 and Enzyme Activities
- Effect of curcumin on acidic pH-induced expression of IL-6 and IL-8 in human esophageal epithelial cells (HET-1A): role of PKC, MAPKs, and NF-κB
- Curcumin Attenuates CFA Induced Thermal Hyperalgesia by Modulation of Antioxidant Enzymes and Down Regulation of TNF-α, IL-1β and IL-6
- [Reducing effect of curcumin on expressions of TNF-alpha, IL-6 and IL-8 in rats with chronic nonbacterial prostatitis].
- Inhibition of IL-6 Signaling Pathway by Curcumin in Uterine Decidual Cells
- TNF-α, IL-6 and IL-10 expressions, responsible for disparity in action of curcumin against cisplatin-induced nephrotoxicity in rats
- The Effect of Curcumin on TNF-α, IL-6 and CRP Expression in a Model of Polycystic Ovary Syndrome as an Inflammation State
- The functional protective effect and the mechanism of curcumin on IL-6 induced neuronal damage in rat hippocampus
- The polyphenols curcumin and resveratrol effectively block IL-1β and PMA-induced IL-6, IL-8 and VEGF-A expression in human rheumatoid synovial fibroblasts
- Effects of Curcumin on NF-κB、TNF-α and IL-6 in ALI/ARDS Rats Induced by Oleinic Acid
- Curcumin Suppression of IL-6 and IL-8 in Head & Neck Cancer: R115
- Curcumin, both Histone Deacetylase and p300/CBP‐Specific Inhibitor, Represses the Activity of Nuclear Factor Kappa B and Notch 1 in Raji Cells
- Protective effect of curcumin in cisplatin-induced oxidative injury in rat testis: mitogen-activated protein kinase and nuclear factor-kappa B signaling pathways
- Curcumin inhibits metastatic progression of breast cancer cell through suppression of urokinase-type plasminogen activator by NF-kappa B signaling pathways
- Curcumin Inhibits Cell Migration of Human Colon Cancer Colo 205 Cells through the Inhibition of Nuclear Factor kappa B /p65 and Down-regulates Cyclooxygenase-2 and Matrix Metalloproteinase-2 Expressions
- Ameliorative effects of curcumin against renal injuries mediated by inducible nitric oxide synthase and nuclear factor kappa B during gentamicin-induced toxicity in Wistar rats
- Liposome-encapsulated curcumin suppresses neuroblastoma growth through nuclear factor-kappa B inhibition
- Hydrogel based oil encapsulation for controlled release of curcumin by using a ternary system of chitosan, kappa-carrageenan, and carboxymethylcellulose sodium salt
- Curcumin prevents shock-wave lithotripsy-induced renal injury through inhibition of nuclear factor kappa-B and inducible nitric oxide synthase activity in rats
- The effect of the NF-kappa B inhibitors curcumin and lactacystin on myogenic differentiation of rhabdomyosarcoma cells
- Curcumin attenuates IL-1β-induced endothelial lipase expression via blocking NF-κB pathway
- Response to “Inhibition of p300 and nuclear factor-[kappa]B by curcumin and its role in diabetic nephropathy”
- Curcumin inhibits mitogen stimulated lymphocyte proliferation, NF-kappa-B activation and IL-2 signaling
- Cerebroprotective role of Tetrahydro Curcumin in hyperhomocysteinemic ischemic mice by regulating NF-kappa B
- Combinatorial effect of curcumin and tumor necrosis factor-α-related apoptosis-inducing ligand (TRAIL) in induction of apoptosis via inhibition of nuclear factor kappa activity and enhancement of caspase-3 activity in chronic myeloid cells: An In-vitro study
- Effect of curcumin on keloid fibroblasts cycle and nuclear factor-kappa B signaling pathways

- The flavonoid cyanidin blocks binding of the cytokine interleukin-17A to the IL-17RA subunit to alleviate inflammation in vivo
- Cyanidin-3-O-β-glucoside attenuates allergic airway inflammation by modulating the IL-4Rα-STAT6 signaling pathway in a murine asthma model
- Anthocyanin-Rich Black Currant Extract and Cyanidin-3-O-Glucoside Have Cytoprotective and Anti-Inflammatory Properties
- Anti-inflammatory effects of black rice, cyanidin-3-O-β-d-glycoside, and its metabolites, cyanidin and protocatechuic acid
- Cyanidin-3-O-β-D-Glucopyranoside Concentrated Materials from Mulberry Fruit Have a Potency to Protect Erectile Function by Minimizing Oxidative Stress in a Rat Model of Diabetic Erectile Dysfunction
- Cyanidin-3-O-β-glucoside inhibits LPS-induced expression ofinflammatory mediators through decreasing IκBα phosphorylation in THP-1 cells
- Cyanidin-3-Glucoside Suppresses Cytokine-InducedInflammatoryResponse in Human Intestinal Cells: Comparison with 5-Aminosalicylic Acid
- Anti-inflammatory protection afforded by cyanidin-3-glucoside and resveratrol in human intestinal cells via Nrf2 and PPAR-γ: Comparison with 5-aminosalicylic acid
- Cyanidin-3-glucoside suppresses Th2 cytokinesand GATA-3 transcription factor in EL-4 T cells
- Cyanidin-3-O-β-glucoside inhibits lipopolysaccharide-inducedinflammatory response in mouse mastitis model
- Cyanidin-3-O-Glucoside Ameliorates Lipopolysaccharide-Induced Injury Both In Vivo and In Vitro Suppression of NF-κB and MAPK Pathways
- Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-κB signaling pathways in SKH-1 hairless mice skin
- Anti-aging Effectsof Cyanidin under a Stress-Induced Premature Senescence Cellular System
- Effects of Cyanidin-3-glycoside on PMA/Ionomycin-induced Th2 Cytokine Productions in RBL-2H3 Cells
- Inhibition of Carrageenan-Induced Acute Inflammation in Mice by Oral Administration of Anthocyanin Mixture from Wild Mulberry and Cyanidin-3-Glucoside
- [Antioxidant and anti-inflammatory effects of cyanidin from cherries on rat adjuvant-induced arthritis].
- Cyanidin-3-glucoside attenuates angiotensin II-induced oxidative stress and inflammation in vascular endothelial cells
- Synergistic effect of atorvastatin and Cyanidin-3-glucoside on angiotensin II-induced inflammation in vascular smooth muscle cells
- Protective Effects of Cyanidin‐3‐O‐β‐glucopyranoside Against UVA‐induced Oxidative Stress in Human Keratinocytes
- Protection of cyanidin-3-glucoside against oxidative stress induced by acrylamide in human MDA-MB-231 cells
- Dietary cyanidin 3‐O‐β‐d‐glucoside increases ex vivo oxidation resistance of serum in rats
- Cyanidin-3-glucoside isolated from mulberry fruit protects pancreatic β-cells against oxidative stress-induced apoptosis
- Synergistic Effect of Cyanidin and PPAR Agonist against Nonalcoholic Steatohepatitis-Mediated Oxidative Stress-Induced Cytotoxicity through MAPK and Nrf2 Transduction Pathways
- Effect of cyanidin 3-O-β-glucopyranoside on hepatic stellate cell proliferation and collagen synthesis induced by oxidative stress
- Oxidation products of cyanidin 3‐O‐β‐d‐glucoside with a free radical initiator
- Neuroprotective effects of cyanidin against Aβ-induced oxidative and ER stress in SK-N-SH cells
- Cyanidin-3-O-Glucoside Modulates the In VitroInflammatory Crosstalk between Intestinal Epithelial and Endothelial Cells
- Cyanidin and Cyanidin-3-O-β-D-glucoside Suppress the Inflammatory Responsesof Obese Adipose Tissue by Inhibiting the Release of Chemokines MCP-1 and MRP-2
- Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice
- Antioxidant and anti-inflammatory activities of cyanidin-3-glucoside and cyanidin-3-rutinoside in hydrogen peroxide and lipopolysaccharide treated RAW264.7 cells (830.23)
- Cyanidin 3-glucoside protects 3T3-L1 adipocytes against H2O2- or TNF-α-induced insulin resistance by inhibiting c-Jun NH2-terminal kinase activation
- Cyanidin-3-O-glucoside Protection against TNF-α-Induced Endothelial Dysfunction: Involvement of Nuclear Factor-κB Signaling
- Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation
- Inhibitory effect of polyphenol cyanidin on TNF-α-induced apoptosis through multiple signaling pathways in endothelial cells
- Cyanidin-3-glucoside suppresses TNF-α-induced cell proliferation through the repression of Nox activator 1 in mouse vascular smooth muscle cells: involvement of the STAT3 signaling
- Cyanidin-3-glucoside Inhibits TNF-α-Induced Mouse Vascular Smooth Muscle Cells Proliferation through Suppression of STAT3 Activation
- Preventive effects of astragaloside IV and its active sapogenin cycloastragenol on cardiac fibrosis of mice by inhibiting the NLRP3 inflammasome
- Evaluation of a novel anti-aging topical formulation containing cycloastragenol, growth factors, peptides, and antioxidants.
- Biocatalysis of Cycloastragenol by Syncephalastrum racemosum and Alternaria alternata to Discover Anti‐Aging Derivatives
- Microbial transformation of the anti-aging agent cycloastragenol by Mucor racemosus
- Cycloastragenol ameliorates experimental heart damage in rats by promoting myocardial autophagy via inhibition of AKT1-RPS6KB1 signaling
DHEA
- Pharmacology and immune modulating properties of 5-androstene-3β,7β,17β-triol, a DHEA metabolite in the human metabolome
- Serum Dehydroepiandrosterone (DHEA) and DHEA Sulfate Are Negatively Correlated with Serum Interleukin-6 (IL-6), and DHEA Inhibits IL-6 Secretion from Mononuclear Cells in Man in Vitro: Possible Link between Endocrinosenescence and Immunosenescence
- Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty
- Plasma concentration of interleukin 6 (IL-6), and its relationship with circulating concentration of dehydroepiandrosterone sulfate (DHEA-S) in patients with chronic idiopathic urticaria
- Increased serum levels of dehydroepiandrosterone (DHEA) and interleukin-6 (IL-6) in women with mild to moderate Alzheimer’s disease
- Dehydroepiandrosterone suppresses interleukin 10synthesis in women with systemic lupus erythematosus
- Regulation of Murine Interleukin-10 Production by Dehydroepiandrosterone
- Activation of Immune Function by Dehydroepiandrosterone (DHEA) in Age-Advanced Men
- Adrenal Hormone Modulation of Type 1 and Type 2 Cytokine Production by Spleen Cells: Dexamethasone and Dehydroepiandrosterone Suppress Interleukin-2, Interleukin-4, and Interferon-γ Productionin Vitro
- Regulation of murine lymphokine production in vivo II. Dehydroepiandrosterone is a natural enhancer of interleukin 2 synthesis by helper T cells
- Changes in Dehydroepiandrosterone (DHEA) and DHEA-Sulfate Plasma Levels during Experimental Endotoxinemia in Healthy Volunteers
- Biochemical changes in the cervical tissue of rabbit induced by interleukin-8, interleukin-1βdehydroepiandrosterone sulphate and prostaglandin E2: a comparative study
- Effect of dehydroepiandrosterone on lipopolysaccharide-induced interleukin-6 production in DH82 cultured canine macrophage cells
- DHEA-Slevels in hypopituitaric patients with severe GH deficiency are strongly reduced across lifespan.Comparison with IGF-I levels before and during rhGH replacement
- Effects of 2-deoxyglucose and dehydroepiandrosterone on intracellular NAD+ level, SIRT1 activity and replicative lifespan of human Hs68 cells
- Physiological Concentrations of DHEA in Human Hair
- Hydroxylation of dehydroepiandrosterone in human scalp hair folhcles
- Metabolic alterations of DHEA and cholesterol sulphates in the hair of patients with acne measured by liquid chromatography–mass spectrometry
- Diurnal and Seasonal Cortisol, Testosterone, and DHEA Rhythms in Boys and Girls during Puberty
- Role of Dehydroepiandrosterone and Dehydroepiandrosterone Sulfate for the Maintenance of Axillary Hair in Women
- Cytokine Dysregulation and Increased Oxidation Is Prevented by Dehydroepiandrosterone in Mice Infected with Murine Leukemia Retrovirus
- Dehydroepiandrosterone (DHEA) replacement decreases insulin resistance and lowers inflammatorycytokines in aging humans
- Modulation of Cytokine Production by Dehydroepiandrosterone (DHEA) Plus Melatonin (MLT) Supplementation of Old Mice
- The endotoxin-induced increase of cytokines is followed by anincrease of cortisol relative to dehydroepiandrosterone (DHEA) inhealthy male subjects
- The Effects of Dehydroepiandrosterone (DHEA) on In Vitro Spleen Cell Proliferation and Cytokine Production
- Altered Cortisol/DHEA Ratio in Tuberculosis Patients and its Relationship with Abnormalities in the Mycobacterial‐driven Cytokine Production by Peripheral Blood Mononuclear Cells
- Dehydroepiandrosterone may be one of the regulators of cytokine production in atopic dermatitis
- The effects of dexamethasone and dehydroepiandrosterone (DHEA) on cytokines and receptor expression in a human osteoblastic cell line: Potential steroid-sparing role for DHEA
- Preliminary studies on the effect of dehydroepiandrosterone (DHEA) on both constitutive and phytohaemagglutinin (PHA)‐inducible IL‐6 and IL‐2 mRNA expression and cytokine production in human spleen mononuclear cell suspensions in vitro
- Role of androgens in dhea-induced rack1 expression and cytokinemodulation in monocytes
- Low cortisol, high DHEA, and high levels of stimulated TNF‐α, and IL‐6 in women with PTSD
- The influence of age and gender on serum dehydroepiandrosterone sulphate (DHEA-S), IL-6, IL-6 soluble receptor (IL-6 sR) and transforming growth factor beta 1 (TGF-β1) levels in normal healthy blood donors
- IL-6, DHEA and the ageing process
- Activity of Lymphocyte Subpopulations in Polymicrobial Sepsis and DHEA Treatment in IL-6 Knockout Mice
- Low levels of cortisol and sIgA, high levels of DHEA-S, and high stimulated levels of TNF-α and IL-6 in women with PTSD
- DHEA Can Inhibit the Proliferation of Myeloma Cells and the IL-6 Production of Bone Marrow Mononuclear Cells from the Myeloma Patients.
- DHEA MODULATES CYTOKINE EXPRESSION VIA IL-6 IN A MULTIPLE
- 7018 POSTER Evaluation of serum IL-6, DHEA and DHEAS levels in comparison with two conventional metastatic markers in melanoma
- Dehydroepiandrosterone (DHEA) inhibits IL-6 secretion from monocytes and peripheral blood mononuclear cells (PBL) in man
- DHEA-dependent and organ-specific regulation of TNF-α mRNA expression in a murine polymicrobial sepsis and trauma model
- Are alterations of lymphocyte subpopulations in polymicrobial sepsis and DHEA treatment mediated by the tumour necrosis factor (TNF)‐α receptor (TNF‐RI)? A study in TNF‐RI (TNF‐RI–/–) knock‐out rodents
- DHEA wirkt protektiv bei einer experimentellen polymikrobiellen Sepsis durch CLP — besteht eine pathogenetische Bedeutung des TNF-α?
- IL-6 vermittelt protektive Effekte des DHEA bei einer polymikrobiellen Sepsis

- In vitro antifungal activity of dragon’s blood from Croton urucurana against dermatophytes
- Observation of the Effect of Dragon’s Blood in Treating Moist Dermatitis Resulted from Radioactive Treatment of Nasopharyngeal Carcinoma
- Dragon’s Blood Inhibits ChronicInflammatory and Neuropathic Pain Responses by Blocking the Synthesis and Release of Substance P in Rats
- Effect of Resina Draconis on the Repair of SkinDefect Treated with Tissue Engineering Skin
- Dragon’s Blood ointment promoting acute skin wound healing in rat
- Effect of Sanguis draconis (a dragon’s blood resin) on streptozotocin- and cytokine-induced β-cell damage, in vitro and in vivo
- Sanguis draconis, a Dragon’s Blood Resin, Attenuates High Glucose-Induced Oxidative Stress and Endothelial Dysfunction in Human Umbilical Vein Endothelial
- Suppression of lipopolysaccharide-induced of inducible nitric oxide synthase and cyclooxygenase-2 by Sanguis Draconis, a dragon’s blood resin, in RAW 264.7 cells
- Dragon’s blood and its extracts attenuate radiation-induced oxidative stress in mice
- Immunomodulatory Activity and Chemical Characterisation of Sangre de Drago (Dragon’s Blood)from Croton lechleri
- Ethylacetate extract from DraconisResinainhibits LPS-inducedinflammatory responses in vascular smooth muscle cells and macrophages via suppression of ROS production
- Mutagenic and antioxidant activities of Croton lechleri sap in biological systems
- Studies on anti-inflammation and analgesic effects of dragon’s blood
- Anti-inflammatory Flavan-3-ol-dihydroretrochalcones from Daemonorops draco
- Anti-Inflammatory And Analgesic Effects Of Ethanol Extract Of Dracaena Cinnabari Balf, As Endemic Plant In Yemen
- Dragon’s Blood May Have Radioprotective Effects in Radiation-Induced Rat Brain Injury
- Dragon’s blood dropping pills have protective effects on focal cerebral ischemia rats model
- Review of Sangre de Drago (Croton lechleri) – A South American Tree Sap in the Treatment of Diarrhea, Inflammation, Insect Bites, Viral Infections, and Wounds: Traditional Uses to Clinical Research
- Effects of Sangre de Drago from Croton lechleri Muell.-Arg. on the production of active oxygen radicals
- Inhibition of Neurogenic Inflammation by the Amazonian Herbal Medicine Sangre de Grado
- The Effect of ProstaglandinF_(2a) (PGF_(2a)) in the Blood Plasma and Interleukin-1?(IL-1?) in the Blood Serum of Endometriosis in the Rats Treated with Complex Prescription Dragon’s Blood Plaster

Eclipta Prostrata
- Antimicrobial activity and cytotoxicity of Eclipta prostrata
- Anti-inflammatory activity of methanolic extract of Eclipta prostrata L. (Astearaceae)
- Ethnopharmacological uses, phytochemistry, biological activities, and biotechnological applications of Eclipta prostrata
- Antidermatophytic activity of Eclipta prostrata L. against human infective Trichophyton and Microsporum spp.
- Three new olean-type triterpenoid saponins from aerial parts of Eclipta prostrata (L.)
- Bioactive triterpenoids, antimicrobial, antioxidant and cytotoxic activities of Eclipta prostrata Linn
- Simultaneous extraction, identification and quantification of phenolic compounds in Eclipta prostrata using microwave-assisted extraction combined with HPLC–DAD–ESI–MS/MS
- Potent Antioxidativeand UVB Protective Effect of Water Extract of Eclipta prostrata L.
- Eclipta prostrata (L.) L. (Asteraceae)–an eco-friendly natural hair dye
- Use of Eclipta prostrataand other PPAR-gamma inhibitors in cosmeticsand compositions thereof
- IN VITROEVALUATION OF TOTAL PHENOLICS AND ANTIOXIDANT ACTIVITIES OF WITHANIA SOMNIFERA, ECLIPTA PROSTRATALAND GOSSYPIUM HERBASCEUML
- PHYTOCHEMICAL SCREENING AND ANTIOXIDANTPOTENTIAL OF ECLIPTA PROSTRATA (L) L-A VALUABLE HERB
- Selected PLants used as hair tonic
- Effects of Herbal Extracts on Hair Growthin an Animal Model of C3H/HeJ Mice
- In-Vitro Study of Anthelmintic Activity of Eclipta prostrata(L) y various Extracts
- Clinical Trials of Eclipta Prostrata (L.)L as Hepatoprotective and Their Market Formulations
- Echinocystic Acid Isolated from Eclipta prostrataSuppresses Lipopolysaccharide-Induced iNOS, TNF-α, and IL-6 Expressions via NF-κB Inactivation in RAW 264.7 Macrophages
- Osteoprotective Effect of Echinocystic Acid, a Triterpone Component from Eclipta prostrata, in Ovariectomy-Induced Osteoporotic Rats
- Ecliptamines A–D, four new guanidine alkaloids from Eclipta prostrata L.
- Eclipta prostrata Improves DSS-Induced Colitis through Regulation of Inflammatory Response in Intestinal Epithelial Cells
- Enhancement of Skin Anti-Inflammatory Activities of Eclipta prostrata L. from the Ultrasonic Extraction Process
- Effect of Eclipta prostrata on 11Beta-Hydroxysteroid Dehydrogenase in Rat Liver and Kidney
- The effects of Eclipta Prostrata L.(Ecliptae Herba) on periodontitis rats
- Wedelolactone from Vietnamese Eclipta prostrata (L) L. Protected Zymosan-induced shock in Mice
- A polysaccharide from Eclipta prostrata alleviates experimental autoimmune encephalomyelitis through inhibiting Th17 cells
- Eclipta prostrata promotes the induction of anagen, sustains the anagen phase through regulation of FGF-7 and FGF-5
- Screening of antibacterial and antioxidant activities of leaves of Eclipta prostrata (L)
- Composition and method for stimulating the synthesis of the melanotic pigment of the skin
- Surfactant-assisted Water Extraction of Flavonoids from Eclipta Prostrata L.
- Primary Discussion on the Hemostatic Activity of Aqueous Extracts from the Leaves of Eclipta prostrata L.

- EGCG Attenuates Autoimmune Arthritis by Inhibition of STAT3 and HIF-1α with Th17/Treg Control
- Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis
- Comparative effects of polyphenols from green tea (EGCG) and soybean (genistein) on VEGF and IL-8release from normal human keratinocytes stimulated with the proinflammatory cytokine TNFα
- Green tea EGCG suppresses T cell proliferation through impairment of IL-2/IL-2 receptor signaling
- Combined administration of EGCG and IL-1 receptor antagonist efficiently downregulates IL-1-inducedtumorigenic factors in U-2 OS human osteosarcoma cells
- EGCG downregulates IL-1RI expression and suppresses IL-1-induced tumorigenic factors in human pancreatic adenocarcinoma cells
- Green tea polyphenol (−)-epigallocatechin gallate blocks epithelial barrier dysfunction provoked by IFN-γ but not by IL-4
- (-)-Epigallocatechin-3-gallate inhibits VEGF expression induced by IL-6 via Stat3 in gastric cancer
- ArticleEpigallocatechin-3-gallateamelioratesautoimmune arthritis by reciprocal regulationof T helper-17 regulatory T cells and inhibitionof osteoclastogenesis by inhibitingSTAT3 signaling
- Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1β-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes
- Epigallocatechin gallate, a constituent of green tea, suppresses cytokine-induced pancreatic β-cell damage
- Novel chemotherapeutic and renal protective effects for the green tea (EGCG): Role of oxidative stressand inflammatory-cytokine signaling
- EGCG attenuates pro-inflammatory cytokines and chemokines production in LPS-stimulated L02 hepatocyte
- The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells
- Effects of Varied EGCG and (+)-CatechinConcentrations on Proinflammatory Cytokines mRNA Expression in ConA-Stimulated Primary White Blood Cell Cultures
- Novel immunoregulatory properties of EGCG on reducing inflammation in EAE
- Epigallocatechin-3-gallate protects pro-inflammatory cytokine induced injuries in insulin-producing cells through the mitochondrial pathway
- Targeting of histamine producing cells by EGCG: a green dart against inflammation?
- Legionella pneumophila Replication in Macrophages Inhibited by Selective Immunomodulatory Effects on Cytokine Formation by Epigallocatechin Gallate, a Major Form of Tea Catechins
- Green Tea Polyphenol Epigallocatechin-3-gallate(EGCG) Differentially Inhibits Interleukin-1β-InducedExpression of Matrix Metalloproteinase-1 and -13 in Human Chondrocytes
- Epigallocatechin-3-O-gallate modulates global microRNA expression in interleukin-1β-stimulatedhuman osteoarthritis chondrocytes: potential role of EGCG on negative co-regulation of microRNA-140-3p and ADAMTS5
- Epigallocatechin-3-gallate inhibits angiotensin II and interleukin-6-induced C-reactive protein production in macrophages
- Stimulation of human monocyte and polymorphonuclear cell iodination and interleukin‐1production by epigallocatechin gallate
- Epigallocatechin-3-gallate inhibits interleukin-6-and angiotensin II-induced production of C-reactive protein in vascular smooth muscle cells
- (−)-Epigallocatechin-3-Gallate Prevents Photocarcinogenesis in Mice through Interleukin-12–Dependent DNA Repair
- Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes
- Regulation of interleukin‐1β–induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin‐3‐gallate in rheumatoid arthritis synovial fibroblasts
- Prevention of Ultraviolet Radiation–Induced Immunosuppression by (−)-Epigallocatechin-3-Gallatein Mice Is Mediated through Interleukin 12–DependentDNA Repair
- Epigallocatechin‐3‐gallate selectively inhibits interleukin‐1 β‐induced activation of mitogen activated protein kinase subgroup c‐Jun N‐terminal kinase in human osteoarthritis chondrocytes
- Epigallocatechin Gallate Attenuates Proliferation and Oxidative Stress in Human Vascular Smooth Muscle Cells Induced by Interleukin-1
via Heme Oxygenase-1 - Regulation of Prostaglandin E2 (PGE2)-induced IL-6 production by Epigallocatechin-3-gallate (EGCG) in rheumatoid arthritis synovial fibroblasts
- Epigallocatechin-3-gallate (EGCG) inhibits IL-6-induced CRP synthesis and ameliorates adiponectin expression in Hep3B cells
- Chronic administration of low dose EGCG inhibits TNF-α-induced IL-6 and IL-8 production in rheumatoid arthritis synovial fibroblasts
- Epigallocatechin-3-gallate (EGCG) inhibits IL-1β-induced IL-6 production and cyclooxygenase-2 (COX-2) expression in rheumatoid arthritis synovial fibroblasts in vitro
- Effect of the green tea polyphenol epigallocatechin gallate (EGCG) on growth and IL-6 signalling pathways in malignant plasma cells
- 48th Annual Meeting of the Orthopaedic Research Society Poster No: 0423 EPIGALLOCATECHIN-3-GALLATE (EGCG) INHIBITS THE INFLAMMATORY EFFECTS OF IL-1BETA IN HUMAN CHONDROCYTES.
- Effects of EGCG from green tea on IL-1β -induced expression of MMP-1 and MMP-13, and production of PGE2 and NO on human IVD cells in vitro
- New TNF-α releasing inhibitors as cancer preventive agents from traditional herbal medicine and combination cancer prevention study with EGCG and sulindac or tamoxifen
- EGCG stimulates the recruitment of brite adipocytes, suppresses adipogenesis and counteracts TNF-α-triggered insulin resistance in adipocytes
- EGCG attenuates the inhibitory effect of high TNF-α level on the process of wound healing in dermal fibroblasts
- The roles of caveolin-1 and heme oxygenase-1 in EGCG-mediated protection against TNF-α-induced endothelial inflammation
- Effects of Green Tea and EGCG on IL-8 Inflammatory Responses in LPS and TNF-α-Induced, Differentiated HT-29 Human Colon Cancer Cells
- INHIBITION OF TNF-α-INDUCED CASPASE-3 ACTIVITY BY EPIGALLOCATECHIN-3-GALLATE (EGCG) IN HUMAN ARTICULAR CHONDROCYTES.
- Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease
- Ellagic acid alleviates adjuvant induced arthritis by modulation of pro- and anti-inflammatory cytokines
- Neuroprotective effects of ellagic acid on cuprizone-induced acute demyelination through limitation of microgliosis, adjustment of CXCL12/IL-17/IL-11 axisand restriction of mature oligodendrocytes apoptosis
- Ellagic acid reduces murine schistosomiasis mansoni immunopathology via up-regulation of IL-10 and down-modulation of pro-inflammatory cytokines production
- Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 via an antioxidant mechanism
- Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a murine model of asthma
- Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice
- Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells
- Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV‐B irradiation
- Anti-Inflammatory Effects of Ellagic Acidon Acute Lung Injury Induced by Acid in Mice
- The Modulatory Effect of Ellagic Acid and Rosmarinic Acid on Ultraviolet-B-Induced Cytokine/ChemokineGene Expression in Skin Keratinocyte (HaCaT) Cells
- Anti‐glycative and anti‐inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice
- Differential Inhibitory Effects of the Polyphenol Ellagic Acid on Inflammatory Mediators NF‐κB, iNOS, COX‐2, TNF‐α, and IL‐6 in 1,2‐Dimethylhydrazine‐Induced Rat Colon Carcinogenesis
- A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats
- Ellagic acidinhibits IL-1β-induced cell adhesion molecule expression in human umbilical vein endothelial cells
- Pharmacodynamics of ellagic acid on cardiac troponin-T, lyosomal enzymes and membrane bound ATPases: Mechanistic clues from biochemical, cytokine and in vitro studies
- Ellagic acid and polyphenolics present in walnut kernels inhibit in vitro human peripheral blood mononuclear cell proliferation and alter cytokineproduction
- Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum
- Molecular and biochemical evidence on the protective role of ellagic acid and silybin against oxidative stress-induced cellular aging
- NF-κB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts
- Inhibitory Effect of an Ellagic Acid-Rich Pomegranate Extract on Tyrosinase Activity and Ultraviolet-Induced Pigmentation
- Effects of Oral Administration of Ellagic Acid-Rich Pomegranate Extract on Ultraviolet-Induced Pigmentation in the Human Skin
- Enhancement of radiation-induced oxidative stress and cytotoxicity in tumor cells by ellagic acid
- Reduction of oxidative stress and apoptosis in hyperlipidemic rabbits by ellagic acid
- Ellagic Acid Prevents Cisplatin‐Induced Oxidative Stress in Liver and Heart Tissue of Rats
- Role of Ellagic Acid against Cisplatin‐Induced Nephrotoxicity and Oxidative Stress in Rats
- Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes
- Ellagic acid attenuates oxidative stress on brain and sciatic nerve and improves histopathology of brain in streptozotocin-induced diabetic rats
- The modulatory effects of ellagic acid and vitamin E succinate on TCDD‐induced oxidative stress in different brain regions of rats after subchronic exposure
- Ellagic acid ameliorates isoproterenol induced oxidative stress: Evidence from electrocardiological, biochemical and histological study
- Therapeutic effects of ellagic acid on memory, hippocampus electrophysiology deficits, and elevated TNF-α level in brain due to experimental traumatic brain injury
- ELLAGIC ACID IMPROVED COGNITIVE DEFICIT, BRAIN ELECTROPHYSIOLOGY DECAY AND BRAIN CONTENT OF TNF-α IN RAT WITH TRAUMATIC BRAIN INJURY
- Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 via an antioxidant mechanism
- Ellagic acid protects against LPS-induced acute lung injury through inhibition of nuclear factor kappa B, proinflammatory cytokines and enhancement of interleukin-10
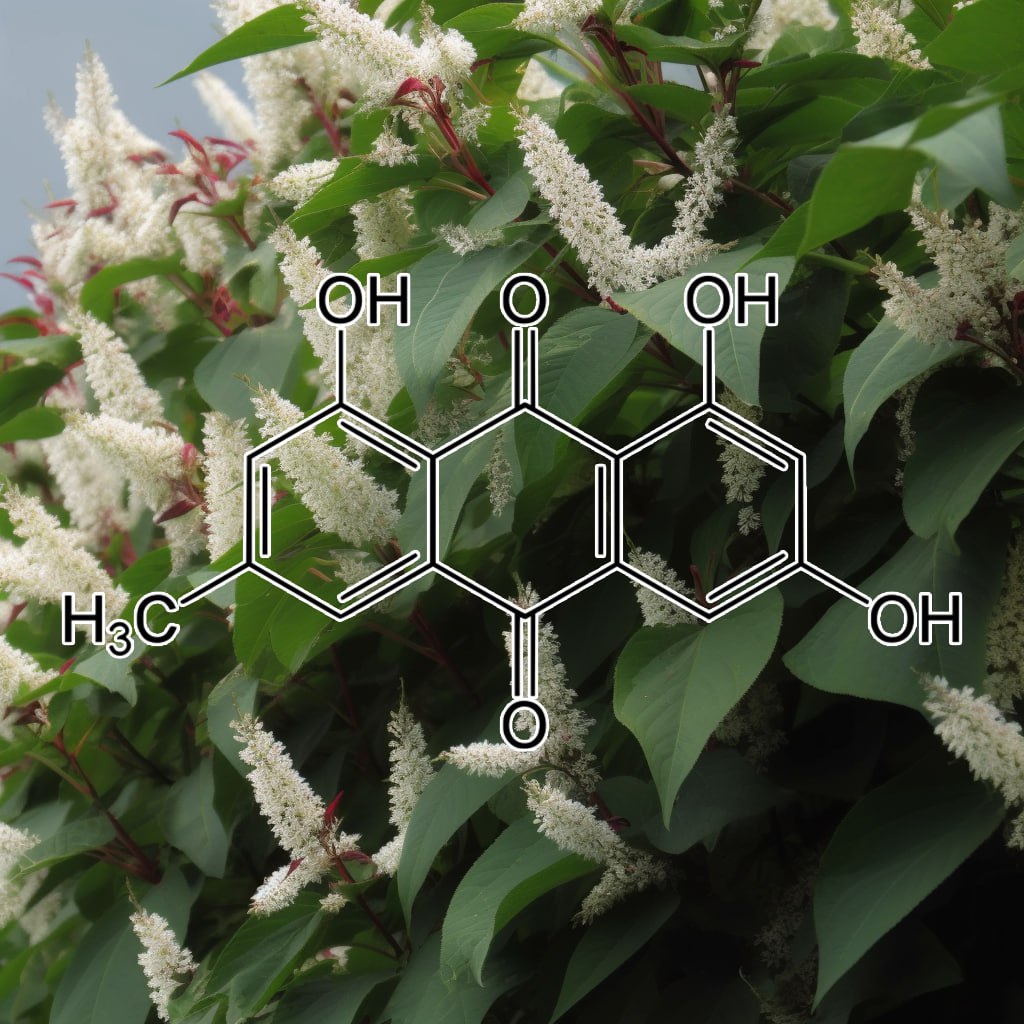
- [Effects of emodin on IL-23/IL-17 inflammatory axis, Th17 cells and viral replication in mice with viral myocarditis].
- Emodin inhibits splenocyte proliferation and inflammation by modulating cytokine responses in a mouse model system
- Emodin inhibited ozone stress-induced airway hyperresponsiveness by regulating epithelial protection mechanisms against injury
- Regulation of cell proliferation, inflammatorycytokineproduction and calcium mobilization in primary human T lymphocytes by emodinfrom Polygonum hypoleucum Ohwi
- Aloe-emodin prevents cytokine-induced tumor cell death: the inhibition of auto-toxic nitric oxide release as a potential mechanism
- Aloe-emodin Affects the Levels of Cytokines and Functions of Leukocytes from Sprague-Dawley Rats
- The influence of emodin and danshensu on monocyte^s secretion of inflammatorycytokines
- The Effects of Emodin on the Expression of Cytokines and Functions of Leukocytes from Sprague-Dawley Rats
- Aloe-emodin from rhubarb (Rheum rhabarbarum) inhibits lipopolysaccharide-inducedinflammatoryresponses in RAW264.7 macrophages
- Emodin Inhibits Proinflammatory Responses and Inactivates Histone Deacetylase 1 in Hypoxic Rheumatoid Synoviocytes
- Emodin suppresses inflammatory responses and joint destruction in collagen-induced arthritic mice
- Emodin inhibits LPS-inducedinflammatory response by activating PPAR-γ in mouse mammary epithelial cells
- Anti-Inflammatory Effect of Emodin via Attenuation of NLRP3 Inflammasome Activation
- Protective effect of emodin against airway inflammation in the ovalbumin-induced mouse model
- Emodin Isolated from Polygoni cuspidati Radix Inhibits TNF-α and IL-6 Release by Blockading NF-κB and MAP Kinase Pathways in Mast Cells Stimulated with PMA Plus A23187
- Emodin inhibits ATP-induced IL-1β secretion, ROS production and phagocytosis attenuation in rat peritoneal macrophages via antagonizing P2X7 receptor
- Emodin ameliorates lipopolysaccharides-induced corneal inflammation in rats
- Chemopreventive effects of emodin and cassiamin B in mouse skin carcinogenesis
- The Molecular Effects of Aloe-Emodin(AE)/Liposome-AE on Human Nonmelanoma Skin Cancer Cells and Skin Permeation
- Ethanol and aloe emodin alter the p53 mutational spectrum in ultraviolet radiation–induced murine skin tumors
- Aloe-emodin inhibits proliferation of adult human keratinocytes in vitro
- Emodin suppresses interleukin-1β inducedmesangial cells proliferation and extracellular matrix production via inhibiting P38 MAPK
- [Interventional effects of emodin on transforming growth factor-beta1/integrin-linked kinase signal way in interleukin-1beta-induced transdifferentiation of rat tubular epithelial-myofibroblasts].
- Emodin mitigates diesel exhaust particles-induced increase in airway resistance, inflammation and oxidative stress in mice
- Critical role of oxidative stress and sustained JNK activation in aloe-emodin-mediated apoptotic cell death in human hepatoma cells
- Protective effects of emodin against cisplatin‐induced oxidative stress in cultured human kidney (HEK 293) cells
- Emodin-Provoked Oxidative Stress Induces Apoptosis in Human Colon Cancer HCT116 Cells through a p53-Mitochondrial Apoptotic Pathway
- Emodin, an anthraquinone derivative, protects against gamma radiation-induced toxicity by inhibiting DNA damage and oxidative stress
- Alteration of subcellular redox equilibrium and the consequent oxidative modification of nuclear factor κB are critical for anticancer cytotoxicity by emodin, a reactive oxygen species-producing agent
- Emodin Prevents Intrahepatic Fat Accumulation, Inflammation and Redox Status Imbalance During Diet-Induced Hepatosteatosis in Rats
- Effect of Emodin on Serum Albumin and TNF-α、IL-6 of Abdominal infection Rats
- Effect of Emodin on TNFα,IL-6 and Apoptosis of Pancreatic Acinar Cells in Severe Acute Pancreatitis in Rats
- Effect of emodin on secretion of TNF_?, IL-1, IL-6, and level of intracellular Ca~(2+) by rat peritoneal macrophage
- Original Article : Emodin Isolated from Polygoni cuspidati Radix Inhibits TNF-α and IL-6 Release by Blockading NF-kB and MAP Kinase Pathways in Mast Cells Stimulated with PMA Plus A23187
- Emodin inhibits TNF α-induced MMP-1 expression through suppression of activator protein-1 (AP-1)
- Emodin inhibits TNF‐α‐induced human aortic smooth‐muscle cell proliferation via caspase‐ and mitochondrial‐dependent apoptosis
- Emodin attenuates TNF-α-induced apoptosis and autophagy in mouse C2C12 myoblasts though the phosphorylation of Akt
- Emodin induced necroptosis in the glioma cell line U251 via the TNF-α/RIP1/RIP3 pathway
- Research on changes of interleukin-1β and TNF- α in acute edematous pancreatitis and the intervention of emodin

Equisetum arvense
- Antinociceptive and anti-inflammatory propertiesof the hydroalcoholic extract of stems from Equisetum arvense L. in mice
- Equisetum arvense (common horsetail) modulates the function of inflammatory immunocompetent cells
- Phytochemistry and pharmacological propertiesof Equisetum arvenseL.
- Antioxidative and Antiproliferative Activities of Different Horsetail (Equisetum arvense L.) Extracts
- Chemical fingerprinting of Equisetum arvenseL. using HPTLC densitometry and HPLC
- The Effect of Equisetum Arvense (Horse Tail) Ointment on Wound Healing and Pain Intensity After Episiotomy: A Randomized Placebo-Controlled Trial
- Antioxidative activities of water extract and ethanol extract from field horsetail (tsukushi) Equisetum arvense L
- Urinary metabolites of flavonoidsand hydroxycinnamic acids in humans after application of a crude extract from Equisetum arvense
- Evaluation of the Subchronic Toxicity of Dietary Administered Equisetum arvense in F344 Rats
- Growth in Vitro of Excised Roots of Equisetum arvense as Modified by Indole-3-Acetic, Indole-3-Butyric, and α-Naphthaleneacetic Acids
- Some Experiments on the Polarity of Spores in Dryopteris erthrosora and Equisetum arvense
- Occurrence of Dermatitis in Rats Fed a Cholesterol Diet Containing Field Horsetail (Equisetum arvense L.)
- Complete plastome sequences of Equisetum arvense and Isoetes flaccida: implications for phylogeny and plastid genome evolution of early land plant lineages
- Composition for treating hair and scalp and method for preparing same
- Cytological and proteomic analyses of horsetail (Equisetum arvense L.) spore germination
- Microwave-assisted extraction for the recovery of antioxidants from waste Equisetum arvense
- Silica deposition and ultrastructure in the cell wall of Equisetum arvense: the importance of cell wall structures and flow control in biosilicification?
- Equisetum arvense L. ExtractInduces Antibacterial Activity and Modulates Oxidative Stress, Inflammation, and Apoptosis in Endothelial Vascular Cells Exposed to Hyperosmotic Stress
- The Effects of Fermented Equisetum arvense on Moisturizing Capabilities in HaCaT Keratinocytes
- Physiological Activities of Extracts from Vegetative Stems of Equisetum arvense
- In Vitro Anti-inflammatory Effects of Equisetum arvenseAre Not Solely Mediated by Silica
- Composition and antimicrobial activity of Equisetum arvense L. essential oil
- Equisetum arvense hydromethanolic extracts in bone tissue regeneration: in vitro osteoblastic modulation and antibacterial activity
- Analysis of Phenolic Compounds Composition by HPLC andAssessment of Antioxidant Capacity in Equisetum arvense L. Extracts
- Evaluation of the efficacy of dietary supplements based on Equisetum arvense, Soy Isoflavones, Lactoferrin and vitamin D3 on the control of climacteric symptoms
- Radical scavenging and antimicrobial activity of horsetail (Equisetum arvense L.) extracts
- Consecutive Production of Hydroalcoholic Extracts, Carbohydrates Derivatives and Silica Nanoparticles from Equisetum arvense
- Three 2-oxoglutarate-dependent dioxygenase activities of Equisetum arvense L. forming flavone and flavonol from (2S)-naringenin
- The Saccharomyces cerevisiae transcriptome as a mirror of phytochemical variation in complex extracts of Equisetum arvense from America, China, Europe and India
- Study of acute hepatotoxicity of Equisetum arvense L. in rats
- Assessing the role of the transcription factor nuclear factor kappa B (NF-kB) in the anti-inflammatory activity of EQUISETUM ARVENSE (COMMON HORSETAIL) extracts

- Fermented Barley Extract Suppresses the Development of Atopic Dermatitis-Like Skin Lesions in NC/Nga Mice, Probably by Inhibiting Inflammatory Cytokines
- Fermented Barley Extract Supplementation Maintained Antioxidative Defense Suppressing Lipopolysaccharide-InducedInflammatoryLiver Injury in Rats
- Effects of Lactobacillus-fermented barley on intestinal morphology, cytokine gene expression, and fecal microbiota in weaned pigs challenged with Escherichia coli K88+
- Dietary supplementation with a fermented barleyand soybean mixture attenuates UVB-induced skin aging and dehydration in hairless mouse skin
- Protective Role of Antioxidant Huskless Barley Extracts on TNF-α-Induced Endothelial Dysfunction in Human Vascular Endothelial Cells

- Ferulic Acid Induces Th1 Responses by Modulating the Function of Dendritic Cells and Ameliorates Th2-Mediated Allergic Airway Inflammation in Mice
- Ferulic acid supresses Th2 immune response and prevents remodeling in ovalbumin-induced pulmonary allergy associated with inhibition of epithelial-derived cytokines
- Ferulic acid inhibits interleukin 17‐dependent expression of nodal pathogenic mediators in fibroblast‐like synoviocytes of rheumatoid arthritis
- Ferulic Acid Stabilizes a Solution of Vitamins C and E and Doubles its Photoprotection of Skin
- FA15, a hydrophobic derivative of ferulic acid, suppressesinflammatory responses and skin tumorpromotion: comparison with ferulic acid
- A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damagecaused by ultraviolet irradiation
- Protective effects of a topical antioxidant mixture containing vitamin C, ferulic acid, and phloretin against ultraviolet‐induced photodamage in human skin
- Protective effect of ferulic acid on 7,12-dimethylbenz[a]anthracene-induced skin carcinogenesis in Swiss albino mice
- Ferulic acid, a phenolic phytochemical, inhibits UVB-induced matrix metalloproteinases in mouse skin via posttranslational mechanisms
- A comparison of skin delivery of ferulic acid and its derivatives: Evaluation of their efficacy and safety
- Skin Delivery of Ferulic Acid from Different Vesicular Systems
- Inhibitory effect of topical application of polymerized ferulic acid, a synthetic lignin, on tumor promotion in mouse skin two-stage tumorigenesis
- Inhibitory Effect of Ferulic Acid and Isoferulic Acid on Murine Interleukin-8 Production in Response to Influenza Virus Infections in vitro and in vivo
- INHIBITORY EFFECT OF FERULIC ACID ON MACROPHAGEINFLAMMATORY PROTEIN-2 PRODUCTION IN A MURINE MACROPHAGE CELL LINE, RAW264.7
- Ferulic acid inhibits neuro-inflammationin mice exposed to chronic unpredictable mild stress
- Attenuation of chronic neuroinflammation by a nitric oxide‐releasing derivative of the antioxidant ferulic acid
- Promising role of ferulic acid, atorvastatin and their combination in ameliorating high fat diet-induced stressin mice
- Iron-induced oxidative damage and apoptosis in cerebellar granule cells: attenuation by tetramethylpyrazine and ferulic acid
- In vivo protective effects of ferulic acid ethyl ester against amyloid‐beta peptide 1–42‐induced oxidative stress
- In vivo protection of synaptosomes by ferulic acid ethyl ester (FAEE) from oxidative stress mediated by 2,2-azobis(2-amidino-propane)dihydrochloride (AAPH) or Fe2+/H2O2: Insight into mechanisms of neuroprotection and relevance to oxidative stress-related neurodegenerative disorders
- Ferulic acid inhibits oxidative stress and cell death induced by Ab oligomers: Improved delivery by solid lipid nanoparticles
- In vitro and in vivo antioxidant properties of ferulic acid: A comparative study with other natural oxidation inhibitors
- Ferulic acid inhibits UV-B–induced oxidative stress in human lymphocytes
- Influence of ferulic acid on γ-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes
- Ferulic Acid Protects Human Umbilical Vein Endothelial Cells from Radiation Induced Oxidative Stress by Phosphatidylinositol 3-Kinase and Extracellular Signal-Regulated Kinase Pathways
- Treatment with ferulic acid to rats with streptozotocin-induced diabetes: effects on oxidative stress, pro-inflammatory cytokines, and apoptosis in the pancreatic β cell
- Redox regulation of cellular stress response by ferulic acid ethyl ester in human dermal fibroblasts: role of vitagenes
- Protective effect of ferulic acid ethyl ester against oxidative stress mediated by UVB irradiation in human epidermal melanocytes
- Ferulic acid protects lymphocytes from radiation-predisposed oxidative stressthrough extracellular regulated kinase
- Ferulic Acid: a Natural Antioxidant Against Oxidative Stress Induced by Oligomeric A-beta on Sea Urchin Embryo
- Aqueous Extract of Tomato (Solanum lycopersicum L.) and Ferulic Acid Reduce the Expression of TNF-α and IL-1β in LPS-Activated Macrophages
- The molecular events behind ferulic acid mediated modulation of IL-6 expression in LPS-activated Raw 264.7 cells
- Ferulic acid ameliorates radiation induced duodenal inflammation by modulating nuclear factor Kappa beta-nuclear factor-2 erythroid related factor-2 cross talk


- A Marine Carotenoid, Fucoxanthin, Induces Regulatory T Cells and Inhibits Th17 Cell Differentiation in Vitro
- Comparative effect of fucoxanthin and vitamin C on oxidative and functional parameters of human lymphocytes
- The Suppressive Effect of a Marine Carotenoid, Fucoxanthin, on Mouse Ear Swelling through Regulation of Activities and mRNA Expression of Inflammation-associated Enzymes
- Protective Effect of Fucoxanthin against UVB-Induced SkinPhotoaging in Hairless Mice
- Anti‐pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules
- Protective and therapeutic effects of fucoxanthin against sunburn caused by UV irradiation
- Chemical Stability and Skin Permeation of Fucoxanthin-Loaded Microemulsions
- FucoxanthinElicits Epigenetic Modifications, Nrf2 Activation and Blocking Transformation in Mouse Skin JB6 P+ Cells
- Fucoxanthin Protects Cultured Human Keratinocytes against Oxidative Stress by Blocking Free Radicals and Inhibiting Apoptosis
- Comparative effects of β-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats
- Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages
- First Evidence for the Anti-inflammatory Activity of Fucoxanthin in High-Fat-Diet-Induced Obesity in Mice and the Antioxidant Functions in PC12 Cells
- Fucoxanthinol, Metabolite of Fucoxanthin, Improves Obesity-Induced Inflammation in Adipocyte Cells
- Fucoxanthin Ameliorates Inflammation and Oxidative Reponses in Microglia
- Anti-Neuroinflammatory Effects of Fucoxanthin via Inhibition of Akt/NF-κB and MAPKs/AP-1 Pathways and Activation of PKA/CREB Pathway in Lipopolysaccharide-Activated BV-2 Microglial Cells
- Anti-inflammatory effect of fucoxanthinderivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage
- Fucoxanthin Inhibits the Inflammation Response in Paw Edema Model through Suppressing MAPKs, Akt, and NFκB
- Protective effects of fucoxanthin against ferric nitrilotriacetate-induced oxidative stressin murine hepatic BNL CL.2 cells
- Fucoxanthin restrains Oxidative stress induced by Retinol Deficiency through Modulation of Na+Ka+-ATPase and Antioxidant Enzyme activities in Rats
- Fucoxanthin restrains oxidative stress induced by retinol deficiency through modulation of Na+Ka+-ATPase and antioxidant enzyme activities in rats
- Protective effect of fucoxanthin isolated from Ishige okamurae against high-glucose induced oxidative stressin human umbilical vein endothelial cells and zebrafish model
- Fucoxanthin restrains oxidative stress induced by retinol deficiency through modulation of Na+Ka+-ATPase and antioxidant enzyme activities in rats
- Fucoxanthin, a marine carotenoid protects cadmium-induced oxidative renal dysfunction in rats
- Fucoxanthin content of Cylindrotheca Closterium and its oxidative stress mediated enhancement
- Fucoxanthin, the constituent of Laminaria japonica, triggers AMPK-mediated cytoprotection and autophagy in hepatocytes under oxidative stress
- Enhancing colour and oxidative stabilities of reduced-nitrite turkey meat sausages during refrigerated storage using fucoxanthin purified from the Tunisian seaweed Cystoseira barbata
- Effects of fucoxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo
- Fucoxanthin inhibits theinflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages
- Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells
- Undaria pinnatifida and Fucoxanthin Ameliorate Lipogenesis and Markers of Both Inflammation and Cardiovascular Dysfunction in an Animal Model of Diet-Induced Obesity
- Inhibition of Ultraviolet B-Induced Expression of the Proinflammatory Cytokines TNF-α and VEGF in the Cornea by Fucoxanthin Treatment in a Rat Model

Ginseng radix
- Promotion of hair growth by ginseng radix on cultured mouse vibrissal hair follicles
- Fructus panax ginseng extract promotes hair regeneration in C57BL/6 mice
- Simultaneous analysis of ginsenosides of various ginseng radix by HPLC
- Carbon dioxide extraction of ginseng root hair oil and ginsenosides
- Comparative Hair Restorer Efficacyof Medicinal Herb on Nude (Foxn) Mice
- Protective effect of Korean Red Ginseng against chemotherapeutic drug-induced premature catagen development assessed with human hair follicle organ culture model
- Compositions and methods of inducing hair growth utilizing Continus coggygria
- Hair–Growth Potential of Ginseng and Its Major Metabolites: A Review on Its Molecular Mechanisms
- Determinations of Trilinolein and 1,2– Dilinoleoyl-3-Oleoyl-Glycerol in Various Panax Ginseng by HPLC
- Panax ginseng extract antagonizes the effect of DKK‑1-induced catagen-like changes of hair follicles
- Experimental Study on the Effects of the Extract of Ginseng Radixet Rhizoma on Hair Growth of C57BL/6J Mice
- Introduction of hemopoiesis by Saenghyuldan a mixture of Ginseng Radix, Paeoniae Radix Alba, and Hominis Placenta Extracts.
- The Effects of Ginseng Radix Rubra on Human Vascular Endothelial Cells
- Effect of Radix Ginseng and Radix Trichosanthis on the Melanogenesis
- Effects of Red Ginseng extract on ultraviolet B‐irradiated skin change in C57BL mice
- Effects of EGb 761 and Korean Red Ginseng on Melanogenesis in B16F10 Melanoma Cells and Protection Against UVB Irradiation in Murine Skin
- Protective effects of Panax ginseng on muscle injury and inflammation after eccentric exercise
- The chemopreventive potential and anti-inflammatory activities of Korean black ginseng in colon26-M3.1 carcinoma cells and macrophages
- Anti-inflammatory mechanism of ginsengsaponins in activated microglia
- COMBINATION EFFECTS OF SHOSAIKOTO (CHINESE TRADITIONAL MEDICINE) AND PREDNISOLONE ON THE ANTI-INFLAMMATORY ACTION
- Panax notoginseng attenuates LPS-induced pro-inflammatory mediators in RAW264.7 cells
- TheEffectsofKoreanRedGinseng(GinsengRadixRubra)onLiverRegenerationafterPartialHepatectomyinDogs
- Pectinase-Processed Ginseng Radix (GINST) Ameliorates Hyperglycemia and Hyperlipidemia in High Fat Diet-Fed ICR Mice
- Hepatoprotective Effects of Chinese Medicinal Herbs: A Focus on Anti-Inflammatory and Anti-Oxidative Activities
- A Natural Compound (Ginsenoside Re) Isolated from Panax ginseng as a Novel Angiogenic Agentfor Tissue Regeneration
- Effects of the Chinese Herbal Medicines Bupleuri radix, Ginseng radix,and Zingiberis rhizoma on Lymphatic Vessel Activity in Rats
- Screening for Anti-inflammatory Activities in Extracts from Korean Herb Medicines
- Anti-Inflammatory and Antimicrobial Effects of a Novel Herbal Formulation (WSY-1075) in a Chronic Bacterial Prostatitis Rat Model
- Effect of Korean Red Ginseng on Testicular Tissue Injuryafter Torsion and Detorsion
- Neuroprotection in Diabetic Encephalopathy
- Ginseng radix induces apoptosis in HL-60 cells and its mechanism as little relation with TNF-α production

- Angiogenic and proliferative effects of the cytokineVEGF in Ehrlich ascites tumor cells is inhibited by Glycyrrhiza glabra
- Licocalchone-C Extracted from Glycyrrhiza GlabraInhibits Lipopolysaccharide-Interferon-γ Inflammation by Improving Antioxidant Conditions and Regulating Inducible Nitric Oxide Synthase Expression
- Glycyrrhiza glabra L. Extract Inhibits LPS-Induced Inflammation in RAW Macrophages
- A Licorice Extract Reduces Lipopolysaccharide‐Induced Proinflammatory Cytokine Secretion by Macrophages and Whole Blood
- Hair Growth Promotant Activity of Petroleum Ether Root Extract of Glycyrrhiza Glabra L (Fabaceae) in Female Rats
- HAIR GROWTH STIMULATING EFFECT AND PHYTOCHEMICAL EVALUATION OF HYDRO-ALCOHOLIC EXTRACT OF GLYCYRRHIZA GLABRA
- GLYCYRRHIZA GLABRA EXTRACT CREAM: EFFECTS ON SKIN PIGMENT “MELANIN”
- Modulation of lipopolysaccharide-induced pro-inflammatory mediators by an extract of Glycyrrhiza glabra and its phytoconstituents
- Protection of Mitochondrial Functions against Oxidative Stresses by Isoflavans from Glycyrrhiza glabra
- Protective activity of Glycyrrhiza glabra Linn. on carbon tetrachloride-induced peroxidative damage
- Anti-convulsant action and amelioration of oxidative stress by Glycyrrhiza glabra root extract in pentylenetetrazole- induced seizure in albino rats
- In vitro studies on protective effect of Glycyrrhiza glabra root extracts against cadmium-induced genetic and oxidative damage in human lymphocytes
- Amelioration of oxidative stress induced by oxidative mutagens and COX-2 inhibitory activity of umbelliferone isolated from Glycyrrhiza glabra L
- In vivoantioxidant and hepatoprotective potential of Glycyrrhiza glabraextract on carbon tetra chloride(CCl4)induced oxidative-stress mediated hepatotoxicity
- Modulation of genotoxicity of oxidative mutagens by glycyrrhizic acid from Glycyrrhiza glabra L.
- New insights into the antioxidant and apoptotic potential of Glycyrrhiza glabra L. during hydrogen peroxide mediated oxidative stress: An in vitro and in silico evaluation
- The Effects of Glycyrrhiza glabra L. extract use with aerobic training on inflammatory factors and cognitive state in elderly with mild cognitive impairment
- Anti-Inflammatory Effects of Glycyrrhiza glabra Linne Extract in a Dextran Sulfate Sodium-Induced Colitis Mouse Model
- Grape seed proanthocyanidin extract (GSPE)differentially regulates Foxp3+ regulatory and IL-17+ pathogenic T cell in autoimmune arthritis
- Proanthocyanidins from the bark of Metasequoia glyptostroboides ameliorate allergic contact dermatitis through directly inhibiting T cells activation and Th1/Th17 responses
- Grape seed proanthocyanidin extract has potent anti-arthritic effects on collagen-induced arthritis by modifying the T cell balance
- Grape Seed Proanthocyanidin Extract Protects Against Carrageenan-Induced Lung Inflammation in Mice Through Reduction of Pro-inflammatory Markers and Chemokine Expressions
- Proanthocyanidins: novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cells
- Grape-Seed Proanthocyanidin Extract as Suppressors of Bone Destruction inInflammatory Autoimmune Arthritis
- Anti-Inflammatory Activity of a Polymeric Proanthocyanidin from Serjania schiedeana
- Grape seed proanthocyanidin extract inhibits interleukin-17-induced interleukin-6 production via MAPK pathway in human pulmonary epithelial cells
- Effects of grape seed proanthocyanidin extract on airway inflammation and Treg/Th17 balance in asthmatic mice
- Anti-inflammatory and Anti-melanogenic Proanthocyanidin Oligomers from Peanut Skin
- Dietary grape-seed proanthocyanidin inhibition of ultraviolet B-induced immune suppression is associated with induction of IL-12
- Grape Seed Proanthocyanidin Extract Attenuates Allergic Inflammation in Murine Models of Asthma
- Protective Effects of Proanthocyanidin on Cerulein-induced Acute Pancreatic Inflammation in Rats
- Grape Seed Proanthocyanidin Extract Attenuates Airway Inflammation and Hyperresponsiveness in a Murine Model of Asthma by Downregulating Inducible Nitric Oxide Synthase
- Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells
- Proanthocyanidin protects against cisplatin‐induced oxidative liver damage through inhibition of inflammation and NF‐κβ/TLR‐4 pathway
- Proanthocyanidin from grape seed extract inhibits airway inflammation and remodeling in a murine model of chronic asthma.
- Grape Seed Proanthocyanidin Extract–Mediated Regulation of STAT3 Proteins Contributes to Treg Differentiation and Attenuates Inflammation in a Murine Model of Obesity-Associated Arthritis
- Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high‐fat diet mice
- Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages
- Gastroprotective Effects of Grape Seed Proanthocyanidin Extracts against Nonsteroid Anti-Inflammatory Drug-Induced Gastric Injury in Rats
- Comparison of the Anti-inflammatory Effects of Proanthocyanidin, Quercetin, and Damnacanthal on Benzo(a)pyrene Exposed A549 Alveolar Cell Line
- Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats
- Effects of Grape Seed Proanthocyanidin Extract on Oxidative Stress Induced by Diabetes in Rat Kidney
- Back‐regulation of six oxidative stress proteins with grape seed proanthocyanidin extracts in rat diabetic nephropathy
- Protection of Primary Glial Cells by Grape Seed Proanthocyanidin Extract against Nitrosative/ Oxidative Stress
- Low molecular proanthocyanidin dietary biofactor Oligonol: Its modulation of oxidative stress, bioefficacy, neuroprotection, food application and chemoprevention potentials
- Proanthocyanidin prevents methotrexate-induced intestinal damage and oxidative stress
- Activin, a Grape Seed-derived ProanthocyanidinExtract, Reduces Plasma Levels of Oxidative Stress and Adhesion Molecules (ICAM-1, VCAM-1 and E-selectin) in Systemic Sclerosis
- Acute and chronic stress-induced oxidativegastrointestinal injury in rats, and the protective ability of a novel grape seed proanthocyanidin extract
- Oral Administration of Grape Seed Proanthocyanidin Extracts Downregulate RAGE Dependant Nuclear Factor- Kappa BP65 Expression in the Hippocampus of Streptozotocin Induced Diabetic Rats
- Effect of Proanthocyanidin on Expression of Nuclear Factor kappa B and Interleukin-6 in Rats after Spinal Cord Injury

- Hesperidin inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice by suppressing Th17 activity
- Antiasthmatic Effects of Hesperidin, a Potential Th2 Cytokine Antagonist, in a Mouse Model of Allergic Asthma
- Hesperidin ameliorates immunological outcome and reduces neuroinflammation in the mouse model of multiple sclerosis
- Hesperidin methyl chalcone inhibits oxidative stress and inflammation in a mouse model of ultraviolet B irradiation-induced skin damage
- Experimental immunology
Differential effect of hesperidin on Th1, Th2, Th17, and proinflammatory cytokines production from splenocyte of Schistosoma mansoni-infected mice - Hesperidin protects gentamicin-induced nephrotoxicity via Nrf2/HO-1 signaling and inhibits inflammation mediated by NF-κB in rats
- Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats
- Hesperidin inhibits expression of hypoxia inducible factor-1 alpha and inflammatory cytokine productionfrom mast cells
- Oral administration of hesperidin, a citrus flavonone, in ratscounteracts the oxidative stress, the inflammatory cytokineproduction, and the hepatotoxicity induced by the ingestion of2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)
- Hesperidin reverses cognitive and depressive disturbances induced by olfactory bulbectomy in mice by modulating hippocampal neurotrophins and cytokine levels and acetylcholinesterase activity
- Hesperidin Suppresses Ovalbumin-Induced Airway Inflammation in a Mouse Allergic Asthma Model
- Hesperidin protects against cyclophosphamide-induced hepatotoxicity by upregulation of PPARγ and abrogation of oxidative stress and inflammation
- Hesperidin Inhibits Inflammatory Response Induced by Aeromonas hydrophila Infection and Alters CD4+/CD8+ T Cell Ratio
- Antiinflammatory Effects of Traditional Korean Medicine, JinPi‐tang and Its Active Ingredient, Hesperidin in HaCaT cells
- Antioxidant and anti-inflammatory potential of hesperidin against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced experimental Parkinson’s disease in mice
- Exposure to Zinc Oxide Nanoparticles Induces Neurotoxicity and Proinflammatory Response: Amelioration by Hesperidin
- HesperidinAlleviates Lipopolysaccharide-Induced Neuroinflammation in Mice by Promoting the miRNA-132 Pathway
- Hesperidin ameliorates UV radiation-induced skin damage by abrogation of oxidative stress andinflammatory in HaCaT cells
- Hesperidin alleviates rat postoperative ileus through anti-inflammation and stimulation of Ca2+-dependent myosin phosphorylation
- Evaluation of antioxidative and anti-inflammatorypotential of hesperidin and naringin on the rat air pouch model of inflammation
- Inhibitory effect of Hesperidin on tumour initiation and promotion in mouse skin
- Preventive effect of hesperidin against inflammation in CD-1 mouse skin caused by tumor promoter.
- Photoprotection by honeybush extracts, hesperidin and mangiferin against UVB-induced skin damage in SKH-1 mice
- Topical hesperidin improves epidermal permeability barrier function and epidermal differentiation in normal murine skin
- Capillary resistance in the skin of rats fed flavone-free and atherogenic diets, and their response to hesperidin-methyl-chalcone.
- Topical formulation containing hesperidin methyl chalcone inhibits skin oxidative stress and inflammation induced by ultraviolet B irradiation
- Topical hesperidin prevents glucocorticoid‐induced abnormalities in epidermal barrier function in murine skin
- Detection of Hesperidin in Orange Skin by the UV-VIS Spectrophotometry
- Hesperidin Suppresses Melanosome Transport by Blocking the Interaction of Rab27A-Melanophilin
- Effect of topical application of hesperidin, aescin and tanshinone on finger nailfold skin microcirculation
- Articels : Hesperidin Improves the IL-6-Mediated Hepatic Insulin Resistance in Hepa-1c1c7 Cells
- Articels : Hesperidin Ameliorates TNF-α-Mediated Insulin Resistance in Differentiated 3T3-L1 Cells
- Effect of hesperidin on lipid peroxidation and TNF-α of alcoholic fatty liver disease in rats
- Effect Of Hesperidin In Bleomycin-Induced Pulmonary Fibrosis In Rats: Critical Role Of NRF-2, TNF-Α, AND IL-1Β
- Influence of Hesperidin Pretreatment on the Expression of TNF-α and IFN-γ in Concanavalin A-induced Acute Liver Injury in Mice
- Influence of Hesperidin on the Expression of TNF-α and IFN-γ in Concanavalin Ainduced Acute Liver Injury in Mice
- Kappa-opioid receptors mediate the antidepressant-like activity of hesperidin in the mouse forced swimming test

Hibiscus rosa-sinensis
- Characterization of the mucilages extracted from hibiscus rosa-sinensis linnand hibiscus mutabilis linn and their skin moisturizing effect
- Antidepressant-like activityof anthocyanidins from Hibiscus rosa-sinensis flowers in tail suspension test and forced swim test
- ANTI-INFLAMMATORY EFFECTS OFHIBISCUS ROSA-SINENSIS L. AND Hibiscus ROSA-SINENSIS VAR.ALBAETHANOL EXTRACTS
- Effect of hydroalcoholic extract of Hibiscus rosa sinensis Linn. leaves in experimental colitis in rats
- A survey on Hibiscus rosa—sinensis, Alcea rosea L. and Malva neglecta Wallr as antibacterial agents
- Antihyperlipidemic Effect ofEthanolic Extract of Hibiscus rosa sinensisFlowers in Hyperlipidemic Rats
- Study on prevention of two-stage skin carcinogenesis by Hibiscus rosa sinensis extract and the role of its chemical constituent, gentisic acid, in the inhibition of tumour promotion response and oxidative stress in mice
- Antioxidant and antibacterial activities of Hibiscus Rosa-sinensis Linn flower extracts
- Mechanisms Involved in Toxicity of Liver Caused by Piroxicam in Mice and Protective Effects of Leaf Extract of Hibiscus Rosa-Sinensis L.
- ANTI-PYRETIC ACTION OF CAULERPA LENTILLIFERA, HIBISCUS ROSA-SINENSIS, AND PIPER SARMENTOSUM AQUEOUS EXTRACT IN MICE
- Evaluation of Antioxidant,Toxicological and wound healing Properties of Hibiscus rosa-sinensisL.(Malvaceae) ethanolic leaves extract on different Experimental animal models
- Polyphenols rich Hibiscus rosa sinensis Linn. petals modulate diabetic stress signalling pathways in streptozotocin-induced experimental diabetic rats
- Gastroprotective effect of flower extracts of Hibiscus rosa sinensis against acute gastric lesion modelsin rodents
- Antibacterial, antioxidant and phytochemical screening of Hibiscus rosa sinensis, Acorus calamus, Cucurbita maxima and Moringa oliefera
- EVALUATION OF ANTI INFLAMMATORY ACTIVITY OF HIBISCUS ROSA SINENSIS Linn. FLOWER EXTRACT IN RATS.
- In-vitro antioxidant activityand phytochemical analysis in extracts of Hibiscus rosa-sinensis stem and leaves
- Hypoglycemic and hypolipidemic activity of Hibiscus rosa sinensis Linn on streptozotocin–induced diabetic rats
- In vivo and in vitro evaluation of hair growth potential of Hibiscus rosa-sinensis Linn.
- The Floral Nectaries of Hibiscus rosa-sinensis: 1. Development of the Secretory Hairs
- Traditional medicinal uses of Hibiscus rosa-sinensis
- Effect of ethanolic extract of Hibiscus rosa sinensis L.,flowers on hair growth in female wistar rats
- Preparation, evaluation and hair growth stimulating activity of herbal hair oil
- Hibiscus rosa sinensis Linn – ‘‘Rudrapuspa’’ : A Review
- Post-Coital Antifertility Activity of Hibiscus rosa-sinensis Linn. Roots
- Comparative Screening of Immunomodulatory Activity of Hydro-alcoholic Extract ofHibiscus rosa sinensis Linn. and Ethanolic Extract of Cleome gynandra Linn
- Cytotoxicity of Hibiscus rosa-sinensis flower extract
- Evaluation of the wound-healing activity of Hibiscus rosa sinensis L (Malvaceae) in Wistar albino rats
- Evaluation of comparative antioxidant potential of four cultivars of Hibiscus rosa-sinensis L. by HPLC-DPPH method
- Effect of Hibiscus rosa sinensis Extract on Hyperproliferation and Oxidative Damage Caused by Benzoyl Peroxide and Ultraviolet Radiations in Mouse Skin
- Preliminary phytochemical and antiulcer studies of Hibiscus rosa sinensis Linn. root extracts
- Functional Jelly Drink as Source of Dietary Fiber and Vitamin C Consisting of Kappa Carrageenan, Konjac Glucomannan and Hibiscus sabdariffa, Linn Extract

Houttuynia cordata
- Effect of Herbal Complex Extract Including Houttuynia cordata Thunb on Hair Growth Promotion in C57BL/6 Mice
- Structures, Components and Functions of Secretory Tissues in Houttuynia cordata
- Antioxidative Effects of Polyphenols in Leaves of Houttuynia cordata on Protein Fragmentation by Copper–Hydrogen Peroxide In Vitro
- Houttuynia cordataThunb: A Review of Phytochemistry and Pharmacology and Quality Control
- Screening of Some Crude Plant Extracts for Their Acaricidal and Insecticidal Efficacies
- Hair growth inhibitors and compositions containing the same
- Determination of As,Hg and 666,DDT of different Houttuynia cordata Accessions
- Antiproliferative Activityand Induction of Apoptosis in Human Melanoma Cellsby Houttuynia cordataThunb Extract
- Antioxidants for Healthy Skin: The Emerging Role of Aryl Hydrocarbon Receptors and Nuclear Factor-Erythroid 2-Related Factor-2
- Decoction and Fermentation of Selected Medicinal Herbs Promote Hair Regrowth by Inducing Hair Follicle Growthin Conjunction with Wnts Signaling
- An Asian traditional herbal complex containing Houttuynia cordata Thunb, Perilla frutescens Var. acuta and green tea stimulates hair growth in mice
- Anti-inflammatory effects of a Houttuynia cordatasupercritical extract
- Flavonoids from the aerial parts of Houttuynia cordataattenuate lung inflammation in mice
- Protective effects from Houttuynia cordata aqueous extract against acetaminophen-induced liver injury
- Houttuynia cordata Thunb inhibits the production of pro‑inflammatory cytokines through inhibition of the NFκB signaling pathway in HMC‑1 human mast cells
- Anti-inflammatory effects of Houttuynia cordatasupercritical extract in carrageenan-air pouch inflammation model
- Anti-Neuroinflammatory Effects of Houttuynia cordata Extract on LPS-Stimulated BV-2 Microglia
- In vitro and Ex vivo Supplementation of Houttuynia cordata Extract and Immunomodulating Effect in Mice
- Protective effects of Houttuynia cordataaqueous extract in mice consuming a high saturated fat diet
- An ethyl acetate fraction derived from Houttuynia cordata extract inhibits the production of inflammatory markers by suppressing NF-кBand MAPK activation in lipopolysaccharide-stimulated RAW 264.7 macrophages
- Houttuynia cordata Thunb reverses oxaliplatin-induced neuropathic pain in rat by regulating Th17/Treg balance
- Paeonia japonica, Houttuynia cordata, and Aster scaber Water Extracts Induce Nitric Oxide and Cytokine Production by Lipopolysaccharide-Activated Macrophages
- Houttuynia cordata aqueous extract attenuated glycative and oxidative stress in heart and kidney of diabetic mice
- Effect of Natural Extracts Mixture from Houttuynia cordataand Ulmus davidiana var. japonica in Mast Cell-Induced Allergic Inflammatory Response
- Vaginal innate immune mediators are modulated by a water extract of Houttuynia cordata Thunb
- Houttuynia cordata suppresses the Aggregatibacter actinomycetemcomitans-induced increase of inflammatory-related genes in cultured human gingival epithelial cells
- Houttuynia cordata water extract suppresses anaphylactic reaction and IgE-mediated allergic response by inhibiting multiple steps of FcεRI signaling in mast cells
- Therapeutic potentials of Houttuynia cordata Thunb. against inflammation and oxidative stress: A review
- Anti-Inflammatory Activity of Houttuynia cordata against Lipoteichoic Acid-Induced Inflammation in Human Dermal Fibroblasts
- Pharmaceutical Impact of Houttuynia Cordata and Metformin Combination on High-Fat-Diet-Induced Metabolic Disorders: Link to Intestinal Microbiota and Metabolic Endotoxemia
- Effects of Volatile Oil and 2-Undecanone from Houttuynia Cordata Thunb. on LPS-TLR4/MD-2-TNF-α Inflammation Signaling Pathway
- Use of hydroxytyrosol as anti-aging agent
- Peracetylated hydroxytyrosol, a new hydroxytyrosol derivate, attenuates LPS-inducedinflammatoryresponse in murine peritoneal macrophages via regulation of non-canonical inflammasome, Nrf2/HO1 and JAK/STAT signaling pathways
- Protective effects of hydroxytyrosol-supplemented refined olive oil in animal models of acute inflammation and rheumatoid arthritis
- Skin delivery of antioxidant surfactants based on gallic acid and hydroxytyrosol
- Inhibition of tyrosinase activity and melanine pigmentation by 2-hydroxytyrosol
- Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells
- Hydroxytyrosol Is the Major Anti-Inflammatory Compound in Aqueous Olive Extracts and Impairs Cytokine and Chemokine Production in Macrophages
- Effects of hydroxytyrosol‐20 on carrageenan‐induced acute inflammation and hyperalgesia in rats
- Suppressive Effects of Hydroxytyrosol on Oxidative Stress and Nuclear Factor-κB Activation in THP-1 Cells
- Hydroxytyrosol prevents metabolic impairment reducing hepatic inflammation and restoring duodenal integrity in a rat model of NAFLD
- Oleic acid, hydroxytyrosol and n-3 fatty acids collectively modulate colitis through reduction of oxidative stress and IL-8 synthesis; in vitro and in vivo studies
- Anti-inflammatory activity of hydroxytyrosol – Inhibition of cytokine production in a parkinson’s model system of neuroinflammation
- Role of the inhibition of oxidative stress and inflammatory mediators in the neuroprotective effects of hydroxytyrosol in rat brain slices subjected to hypoxia reoxygenation
- Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages
- Quercetin and hydroxytyrosolattenuates xanthine/xanthine oxidase-induced toxicity in H9c2 cardiomyocytes by regulation of oxidative stress and stress-sensitive signaling pathways
- Anti-Inflammatory and Antitumor Effects of Hydroxytyrosol but Not Oleuropein on Experimental Glioma In Vivo. A Putative Role for the Renin-Angiotensin System
- Hydroxytyrosol exerts an anti-inflammatory effect by suppressing Toll-like receptor 2 and TLR 2 downstream pathways in Staphylococcus aureus-induced mastitis in mice
- Hydroxytyrosol inhibits the inflammatory response of osteoarthritis chondrocytes via SIRT6-mediated autophagy
- Antiproliferative, Antioxidant and Anti-inflammatoryEffects of Hydroxytyrosol on Human Hepatoma HepG2 and Hep3B Cell Lines
- Influence of olive-derived hydroxytyrosol on the toll-like receptor 4-dependentinflammatory response of mouse peritoneal macrophages
- Hydroxytyrosol modulates the levels of microRNA-9 and its target sirtuin-1 thereby counteracting oxidative stress-induced chondrocyte death
- Hydroxytyrosol and oleuropein from olive leaves: Potent anti-inflammatory andanalgesic activities
- Olive Phenol Hydroxytyrosol Prevents Passive Smoking–Induced Oxidative Stress
- Hydroxytyrosol protects retinal pigment epithelial cells from acrolein‐induced oxidative stress and mitochondrial dysfunction
- Olive oil hydroxytyrosolprotects human erythrocytes against oxidative damages
- Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: activation of Nrf2 and JNK-p62/SQSTM1 pathways
- The olive oil antioxidant hydroxytyrosol efficiently protects against the oxidative stress-induced impairment of the NO• response of isolated rat aorta
- Protective effect of hydroxytyrosoland tyrosol against oxidative stress in kidney cells
- Olive oil hydroxytyrosol reduces toxicity evoked by acrylamide in human Caco-2 cells by preventing oxidative stress
- Protective Effects of Synthetic Hydroxytyrosol Acetyl Derivatives against Oxidative Stress in Human Cells
- The hydroxytyrosol-dependent increase of TNF-α in LPS-activated human monocytes is mediated by PGE2 and adenylate cyclase activation
- Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways
- Inhibition of Th1/Th17 responses via suppression of STAT1 and STAT3 activation contributes to the amelioration of murine experimental colitis by a natural flavonoid glucoside icariin
- Regulation of Th17/Treg function contributes to the attenuation of chronic airway inflammation by icariinin ovalbumin-induced murine asthma model
- A natural flavonoid glucoside icariin inhibits Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis
- A Natural Flavonoid Glucoside, Icariin, Regulates Th17and Alleviates Rheumatoid Arthritis in a Murine Model
- Icariin inhibits LPS-induced cell inflammatory response by promoting GRα nuclear translocation and upregulating GRα expression
- Chondroprotective effects and multi-target mechanisms of Icariin in IL-1 beta-induced human SW 1353 chondrosarcoma cells and a rat osteoarthritis model
- Icariin prevents cytokine‑induced β‑cell death by inhibiting NF‑κB signaling
- Icariin inhibits TNF-α/IFN-γ inducedinflammatoryresponse via inhibition of the substance P and p38-MAPK signaling pathway in human keratinocytes
- Icariin promotes mouse hair follicle growth by increasing insulin‐like growth factor 1 expression in dermal papillary cells
- Icariin induces cell differentiation and cell cycle arrest in mouse melanoma B16 cells via Erk1/2-p38-JNK-dependent pathway
- Effects of Icariin on Hypothalamic-Pituitary-Adrenal Axis Action and Cytokine Levels in Stressed Sprague-Dawley Rats
- Icariin Regulates Expression of Cytokines in Osteoblasts
- Icariin protects against titanium particle-induced osteolysis and inflammatory responsein a mouse calvarial model
- Effects of icariin on cytokine-induced ankylosing spondylitis with fibroblastic osteogenesis and its molecular mechanism
- Attenuation of LPS-induced inflammation by ICT, a derivate of icariin, via inhibition of the CD14/TLR4 signaling pathway in human monocytes
- Icariin Attenuates High-cholesterol Diet Induced Atherosclerosis in Rats by Inhibition of InflammatoryResponse and p38 MAPK Signaling Pathway
- Inhibitory effects of icariin on melanogenesisof human epidermal melanocytes
- Icariin inhibits inflammation via immunomodulation of the cutaneous hypothalamus–pituitary–adrenal axis in vitro
- Icariin Attenuates Interleukin-1β-InducedInflammatory Response in Human Nucleus Pulposus Cells
- [Effects of icariin on inflammation model stimulated by lipopolysaccharide in vitro and in vivo].
- Icariin attenuates LPS-induced acuteinflammatoryresponses: Involvement of PI3K/Akt and NF-κB signaling pathway
- Inhibitory effect of icariin on Ti-inducedinflammatoryosteoclastogenesis
- Icariinprotects murine chondrocytes from lipopolysaccharide-inducedinflammatory responses and extracellular matrix degradation
- Effects of icariin combined with Panax notoginseng saponins on ischemia reperfusion‐induced cognitive impairments related with oxidative stress and CA1 of hippocampal neurons in rat
- Icariin Promotes Angiogenic Differentiation andPrevents Oxidative Stress-Induced Autophagy inEndothelial Progenitor Cells
- Icariin attenuates hypoxia‐induced oxidative stress and apoptosis in osteoblasts and preserves their osteogenic differentiation potential in vitro
- [Icariin reduces mitochondrial oxidative stress injury in diabetic rat hearts].
- Icariinexerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation
- Protective effect of icariin on DNA against radical‐induced oxidative damage
- Icariin attenuated oxidative stressinduced-cardiac apoptosis by mitochondria protection and ERK activation
- STUDY OF SYNERGISTIC EFFECTS OF ICARIIN ON INDUCING IL-2、3、 6
- Icariin inhibits MMP‑1, MMP‑3 and MMP‑13 expression through MAPK pathways in IL‑1β‑stimulated SW1353 chondrosarcoma cells
- Icariin Intervenes in Cardiac Inflammaging through Upregulation of SIRT6 Enzyme Activity and Inhibition of the NF-Kappa B Pathway
- Icariin ameliorates IgA nephropathy by inhibition of nuclear factor kappa b/Nlrp3 pathway
- Icariin improves Fanconi anemia hematopoietic stem cell function through SIRT6-mediated NF-kappa B inhibition
Isorhapontigenin
- Agent for hair deforming treatment
- Phytochemistry and Biological Activity Perspectives of Rheum Species
- Isorhapontigenin, a bioavailable dietary polyphenol, suppresses airway epithelial cell inflammation through a corticosteroid‐independent mechanism
- Cardioprotective effect of resveratrol analogue isorhapontigenin versus omega-3 fatty acids in isoproterenol-induced myocardial infarction in rats
- Effect of isorhapontigenin on respiratory burst of rat neutrophils
- Piceatannol and Its Metabolite, Isorhapontigenin, Induce SIRT1 Expression in THP-1 Human Monocytic Cell Line
- An Isorhapontigenin Tetramer and a Novel Stilbene Dimer from Gnetum hainanense
- The Chinese Herb Isolate Isorhapontigenin Induces Apoptosis in Human Cancer Cells by Down-regulating Overexpression of Antiapoptotic Protein XIAP
- Studies on formic acid-catalyzed dimerization of isorhapontigenin and of resveratrol to tetralins
- Isorhapontigenin, a new resveratrol analog, attenuates cardiac hypertrophy via blocking signaling transduction pathways
- Administration of the Resveratrol Analogues Isorhapontigenin and Heyneanol-A Protects Mice Hematopoietic Cellsagainst Irradiation Injuries
- Quantum Chemical Study on the Antioxidation Mechanism of Piceatannol and Isorhapontigenin toward Hydroxyl and Hydroperoxyl Radicals
- A Theoretical Study on the Antioxidant Activity of Piceatannol and Isorhapontigenin Scavenging Nitric Oxide and Nitrogen Dioxide Radicals
- Pre-clinical Pharmacokinetic and Metabolomic Analyses of Isorhapontigenin, a Dietary Resveratrol Derivative
- [Transport mechanism of isorhapontigenin based on human intestinal Caco-2 cells].
- Induction of miR-137 by Isorhapontigenin (ISO) Directly Targets Sp1 Protein Translation and Mediates Its Anticancer Activity Both In Vitro and In Vivo

Kaempferol
- Dietary Flavonoids as Therapeutics for Preterm Birth: Luteolin and Kaempferol Suppress Inflammation in Human Gestational Tissues In Vitro
- Protective Effects of Kaempferol on Lipopolysaccharide-Induced Mastitis in Mice
- [Inhibitory effect of kaempferol on inflammatory responseof lipopolysaccharide-stimulated human mast cells].
- Dietary Kaempferol Suppresses Inflammation of Dextran Sulfate Sodium-Induced Colitis in Mice
- Protective Effect of Kaempferol on LPS plus ATP-Induced Inflammatory Responsein Cardiac Fibroblasts
- Kaempferol pretreatment modulates systemic inflammation and oxidative stress following hemorrhagic shock in mice
- Kaempferol Attenuates Myocardial Ischemic Injury via Inhibition of MAPK Signaling Pathway in Experimental Model of Myocardial Ischemia-Reperfusion Injury
- Kaempferol Promotes Transplant Tolerance by Sustaining CD4+FoxP3+ Regulatory T Cells in the Presence of Calcineurin Inhibitor
- Kaempferol Inhibits P. intermedia Lipopolysaccharide‐Induced Production of Nitric Oxide Through Translational Regulation in Murine Macrophages: Critical Role of Heme Oxygenase‐1‐Mediated ROS Reduction
- STAT3 and NF-κB are common targets for kaempferol-mediated attenuation of COX-2 expression in IL-6-induced macrophages and carrageenan-induced mouse paw edema
- Inhibition of LPS induced iNOS, COX-2 and cytokines expression by kaempferol-3-O-β-D-sophoroside through the NF-κB inactivation in RAW 264.7 cells [2008]
- Protective effect of kaempferol, a flavonoid widely present in varieties of edible plants, on IL-1β-induced inflammatory response via inhibiting MAPK, Akt, and NF-κB signalling in SW982 cells
- Kaempferol alleviates LPS-induced neuroinflammation and BBB dysfunction in mice via inhibiting HMGB1 release and down-regulating TLR4/MyD88 pathway
- Kaempferol inhibits the production of ROS to modulate OPN–αvβ3 integrin pathway in HUVECs
- Kaempferol impedes IL-32-induced monocyte-macrophage differentiation
- Kaempferol slows intervertebral disc degeneration by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammation responsein BMSCs
- Expression of the xenobiotic metabolizing enzyme cytochrome P450 1B1 alters anti-inflammatory activity of quercetin, kaempferol and taxifolin in macrophage and monocyte (830.25)
- Effect of Cudrania tricuspidata and Kaempferol in Endoplasmic Reticulum Stress-Induced Inflammation and Hepatic Insulin Resistance in HepG2 Cells
- Kaempferol7-O-β-D-glucoside isolated from the leaves of Cudrania tricuspidata inhibits LPS-induced expression of pro-inflammatory mediators through inactivation of NF-κB, AP-1, and JAK-STAT in RAW 264.7 macrophages
- Procyanidin Oligomers Selectively and Intensively Promote Proliferation of Mouse Hair Epithelial Cells In Vitro and Activate Hair Follicle Growth In Vivo
- Hair Growth Promotingand Anticancer Effects of p21-activated kinase 1 (PAK1) Inhibitors Isolated from Different Parts of Alpinia zerumbet
- Inhibitory effectsof quercetin and Kaempferol as two propolis derived flavonoids on Tyrosinase enzyme
- Methods and compounds for reducing allergic reactions to hair dyes
- Mechanism of action of herbs and their active constituents used in hair loss treatment
- Protection of burn-induced skin injuries by the flavonoid kaempferol
- Stimulating effect of kaempferoland six traditional Chinese medicines on activation of tyrosinase
- Method For Preparing Kaempferol-3-0-Rutinoside and Composition of Skin External Application Comprising Thereof
- Kaempferol Regulates Collagen Induced Oxidation of SHP-2 in Platelet Activation
- Antioxidant and Neuroprotective Effects of Kaempferol and Tannic Acid against 6-Hydroxydopamine-induced Oxidative Stress
- Composition for preventing hair loss and stimulating hair growth
- Kaempferol attenuates COX-2 expression in IL-6-induced macrophages and carrageenan-induced mouse paw edema by targeting STAT3 and NF-kB
- Down-regulation of iNOS and TNF-α expression by kaempferol via NF-κB inactivation in aged rat gingival tissues
- Downregulation in the mRNA expression of nuclearhormone receptor liver-X-receptor alpha (LXR-ααααα) byTNF-ααααα is abolished by the antioxidant kaempferol, butnot ascorbic acid, in human hepatocarcinoma HepG2cells
- Nicotinic acid, lauric acid and kaempferol abolish ATP-binding cassette transporter subfamily A member 1 (ABCA1) down-regulation by TNF-αin hepatocarcinoma HepG2 cell line.
- Inhibition of LPS induced iNOS, COX-2 and cytokines expression by kaempferol-3-O-β
-D-sophoroside through the NF−κB
inactivation in RAW 264.7 cells

Kolaviron
- Effects of Kolaviron, the Major Constituent of Garciniakola, on the Histology of the Hypothalamus, Pituitary, and Testes Using Adult Male WistarRats as a Model Organism
- Hair growth compositionsand methods of use
- Oral phenytoin protects against experimental cyclophosphamide-chemotherapy induced hair loss
- Kolaviron-Induced Inhibition of Lung Adencarcinoma (H1299) Cell Growth and Survival via PKA/PI3K Pathway
- Kolaviron, a natural flavonoid from the seeds of Garcinia kola, reduces LPS-induced inflammation in macrophagesby combined inhibition of IL-6 secretion, and inflammatory transcription factors, ERK1/2, NF-κB, p38, Akt, p-c-JUN and JNK
- Kolaviron, a Garcinia biflavonoid complex ameliorates hyperglycemia-mediated hepatic injury in rats via suppression of inflammatory responses
- Inhibition of neuroinflammation in BV2 microglia by the biflavonoid kolavironis dependent on the Nrf2/ARE antioxidant protective mechanism
- Kolaviron protects against benzo[a]pyrene-induced functional alterationsalong the brain-pituitary-gonadal axis in male rats
- Anti-inflammatory effects of kolaviron modulate the expressions of inflammatory marker genes, inhibit transcription factors ERK1/2, p-JNK, NF-κB, and activate Akt expressions in the 93RS2 Sertoli cell lines
- Kolaviron and selenium reduce hydrogen peroxide-induced alterations of the inflammatory response
- The effects of Kolaviron, a Natural Flavonoid from the Seeds of Garcinia Kola on LPS-induced Inflammation in Macrophages
- Kolaviron Reduces Cadmium-induced Cytotoxicity and Production of Reactive Oxygen Species by Suppressing Inflammatory Response
- Neuroprotective role of kolaviron in striatal redo-inflammation associated with rotenone model of Parkinson’s disease
- Effects of kolaviron–a Garcinia kola biflavonoid on biochemical and histological parameters in streptozotocin – induced diabetes and diabetic complications (nephrotoxicity and hepatotoxicity) in male Wistar rats
- Renoprotection of Kolaviron against benzo (A) pyrene-induced renal toxicity in rats
- Kolaviron, a Natural Antioxidant and Anti‐InflammatoryPhytochemical Prevents Dextran Sulphate Sodium‐Induced Colitis in Rats
- Analgesic and anti-inflammatory effects of kaviiron (a Garcinia kola seed extract)
- Kolaviron, a biflavonoid complex of Garcinia kola seeds modulates apoptosis by suppressing oxidative stress and inflammationin diabetes-induced nephrotoxic rats
- Kolaviron Improved Resistance to Oxidative Stress and Inflammationin the Blood (Erythrocyte, Serum, and Plasma) of Streptozotocin-Induced Diabetic Rats
- Anti-inflammatory activities of a kolaviron-inhibition of nitric oxide, prostaglandin E2 and tumor necrosis factor-alpha production in activated macrophage-like cell line.
- Kolaviron Improves Morbidity and Suppresses Mortality by Mitigating Oxido-Inflammation in BALB/c Mice Infected with Influenza Virus
- ANTI‐OXIDANT MECHANISMS OF KOLAVIRON: STUDIES ON SERUM LIPOPROTEIN OXIDATION, METAL CHELATION AND OXIDATIVE MEMBRANE DAMAGE IN RATS
- The Garcinia kola biflavonoid kolaviron attenuates experimental hepatotoxicity induced by diclofenac
- Kolaviron was protective against sodium azide (NaN3) induced oxidative stress in the prefrontal cortex
- Antinociceptive and anti-inflammatory potentials of kolaviron: mechanisms of action
- Garcinia kola seed biflavonoid fraction (Kolaviron), increases longevityand attenuates rotenone-induced toxicity in Drosophila melanogaster
- Chemoprevention of aflatoxin B1-induced genotoxicity and hepatic oxidative damage in rats by kolaviron, a natural biflavonoid of Garcinia kola seeds
- Possible Protective Effect of Kolaviron on CCl4-Induced Erythrocyte Damage in Rats
- Kolaviron Modulates cellular redox status and impairment of membrane proteinactivities induced by potassium bromate (KBrO3) in rats
- Antioxidantand Scavenging Activities of Flavonoid Extract (Kolaviron) of Garcinia kola Seeds

Ligustri fructus
- Antioxidant phenolic profile from ethyl acetate fraction of Fructus Ligustri Lucidi with protection against hydrogen peroxide-induced oxidative damage in SH-SY5Y cells
- Ligustri Lucidi Fructus as a traditional Chinese medicine: a review of its phytochemistry and pharmacology
- Improvement of calcium balance by Fructus Ligustri Lucidi extract in mature female rats was associated with the induction of serum parathyroid hormone levels
- Fructus ligustri lucidi extracts induce human glioma cell death through regulation of Akt/mTOR pathway In Vitro and reduce glioma tumor growth in U87MG xenograft mouse model
- Antioxidant Effect of Fructus Ligustri Lucidi Aqueous Extract in Ovariectomized Rats Is Mediated through Nox4-ROS-NF-κB Pathway
- The Advances in Research on the Pharmacological Effects of Fructus Ligustri Lucidi
- [Effects of mixture of Astragalus membranaceus, Fructus Ligustri lucidi and Eclipta prostrata on immune function in mice].
- Hypoglycemic effect of fructus Ligustri Lucidi
- Analgesic and Anti-Inflammatory Activities of Aqueous Extracts of Fructus Ligustri Lucidi
- Hair growth is promoted by BeauTop via expression of EGF and FGF‑7
- Hair growth effect of traditional Chinese medicine BeauTop on androgenetic alopecia patients: A randomized double-blind placebo-controlled clinical trial
- Decoction and Fermentation of Selected Medicinal Herbs Promote Hair Regrowth by Inducing Hair Follicle Growth in Conjunction with Wnts Signaling

- Prophylactic effects of Lonicera japonica extracton dextran sulphate sodium-induced colitis in a mouse model by the inhibition of the Th1/Th17 response
- P01.12. Prophylactic effects of Lonicera japonica extracton dextran sulfate sodium-induced colitis in a mouse model by inhibition of the Th1/Th17 response
- Luteolin Isolated from the Flowers of Lonicera japonica Suppresses Inflammatory Mediator Release by Blocking NF-κB and MAPKs Activation Pathways in HMC-1 Cells
- Wound repair and anti-inflammatory potential of Lonicera japonica in excision wound-induced rats
- Anti-inflammatory effect of the aqueous extract from Lonicera japonica flower is related to inhibition of NF-kappaB activation through reducing I-kappaBalpha degradation in rat liver.
- Inhibition of Experimental Systemic Inflammation(Septic Inflammation) and Chronic Bronchitis by New Phytoformula BL Containing Broussonetia papyrifera and Lonicera japonica
- Anti-inflammatory effect of Lonicera japonica in proteinase-activated receptor 2-mediated paw edema
- Inhibitory Effect of Aqueous Extract from Lonicera japonica Flower on LPS-inducedInflammatoryMediators in RAW 264.7 Macrophages.
- Flavonoids Isolated from Flowers of Lonicera japonicaThunb. Inhibit Inflammatory Responses in BV2 Microglial Cells by Suppressing TNF‐α and IL‐β Through PI3K/Akt/NF‐kb Signaling Pathways
- Loniceroside C, an Antiinflammatory Saponin from Lonicera japonica
- Antiinflammatory activity of Lonicera japonica
- Lonicera Japonica Inhibits Atopy Dermatitis in NC/Nga Mouse through Regulation of iNOS by NF-κB
Suppression
- Anti-inflammatory effects of luteolin on experimental autoimmune thyroiditis in mice
- Luteolin reduces inflammation in Staphylococcus aureus-induced mastitis by inhibiting NF-κB activation and MMPs expression
- Brain “fog,” inflammationand obesity: key aspects of neuropsychiatric disorders improved by luteolin
- Luteolin alleviates melanogenesis through regulation of oxidative insults
- Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects
- Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model
- Luteolin attenuates airway inflammation by inducing the transition of CD4+CD25– to CD4+CD25+ regulatory T cells
- Luteolin Prevents Solar Radiation-Induced Matrix Metalloproteinase-1 Activation in Human Fibroblasts: A Role for p38 Mitogen-Activated Protein Kinase and Interleukin-20 Released from Keratinocytes
- Luteolin Suppresses Cisplatin-Induced Apoptosis in Auditory Cells: Possible Mediation Through Induction of Heme Oxygenase-1 Expression
- Luteolin Inhibits an Endotoxin-Stimulated Phosphorylation Cascade and Proinflammatory Cytokine Production in Macrophages
- Luteolin inhibits cytokine expression in endotoxin/cytokine-stimulated microglia
- Luteolin suppresses IL-1β-induced cytokinesand MMPs production via p38 MAPK, JNK, NF-kappaB and AP-1 activation in human synovial sarcoma cell line, SW982
- Luteolin Reduces Lipopolysaccharide-induced Lethal Toxicity and Expression of Proinflammatory Moleculesin Mice
- Luteolin Inhibits Hyperglycemia‐Induced Proinflammatory Cytokine Production and Its Epigenetic Mechanism in Human Monocytes
- Antiasthmatic Activity of Luteolin-7-O-glucoside from Ailanthus altissima through the Downregulation of T Helper 2 Cytokine Expression and Inhibition of Prostaglandin E2 Production in an Ovalbumin-Induced Asthma Model
- Luteolin suppresses inflammation-associated gene expression by blocking NF-κB and AP-1 activation pathway in mouse alveolar macrophages
- Anti-Inflammatory and Antipruritic Effects of Luteolin from Perilla (P. frutescens L.) Leaves
- Inhibitory Effect of Perilla Leaf Extract and Luteolin on Mouse Skin Tumor Promotion
- Luteolin inhibits myelin basic protein‐induced human mast cell activation and mast cell‐dependent stimulation of Jurkat T cells
- Flavonoids such as Luteolin, Fisetin and Apigenin Are Inhibitors of Interleukin-4 and Interleukin-13Production by ActivatedHuman Basophils
- Modulatory effects of luteolin on osteoblastic function andinflammatory mediators in osteoblastic MC3T3‐E1 cells
- Luteolin inhibits mast cell-mediated allergic inflammation.
- Chemical characterization and anti-inflammatoryactivity of luteolin glycosides isolated from lemongrass
- Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6
- Inhibitory Effect of Luteolin 4′-O-Glucoside from Kummerowia striata and Other Flavonoids on Interleukin-5 Bioactivity
- Biphasic effects of luteolin on interleukin-1β-inducedcyclooxygenase-2 expression in glioblastoma cells
- Luteolin decreases invasiveness, deactivates STAT3 signaling, and reverses interleukin-6 induced epithelial–mesenchymal transition and matrix metalloproteinase secretion of pancreatic cancer cells
- Stimulated human melanocytes express and release interleukin‐8, which is inhibited by luteolin: relevance to early vitiligo
- Effects of Luteolin on the Release of Nitric Oxide and Interleukin‐6 by Macrophages Stimulated With Lipopolysaccharide From Prevotella Intermedia
- Chlorogenic acid and luteolin synergistically inhibit the proliferation of interleukin-1β-induced fibroblast-like synoviocytes through regulating the activation of NF-κB and JAK/STAT-signaling pathways
- Luteolin suppresses IL-1β-induced cytokines and MMPs production via p38 MAPK, JNK, NF-kappaB and AP-1 activation in human synovial sarcoma cell line, SW982
- Luteolin inhibited the gene expression, production and secretion of MUC5AC mucin via regulation of nuclear factor kappa B signaling pathway in human airway epithelial cells
- Luteolinattenuates adipocyte-derived inflammatory responses viasuppression ofNF-κB/MAPK pathway
- Luteolin attenuates TGF-beta 1-induced epithelial-mesenchymal transition of lung cancer cells by interfering in the PI3K/Akt-NF-kappa B-Snail pathway

- Proanthocyanidins from the bark of Metasequoia glyptostroboides ameliorate allergic contact dermatitis through directly inhibiting T cells activation and Th1/Th17 responses
- Antioxidant Capacity and Proanthocyanidin Composition of the Bark of Metasequoia glyptostroboides
- Proanthocyanidins: novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cells
- The Role of Flavonoids in Inhibiting Th17 Responses in Inflammatory Arthritis
- Flavonoids from metasequoia Glyptostroboides
- Antioxidant and antidermatophytic activities of essential oil and extracts of Metasequoia glyptostroboides Miki ex Hu
- Antioxidant Capacity and Proanthocyanidin Composition of the Bark of Metasequoia glyptostroboides
- Extraction and isolation progress of total flavonoids from Metasequoia glyptostroboides hu et cheng with adsorption method
- Antioxidant, lipid peroxidation inhibition and free radical scavenging efficacy of a diterpenoid compound sugiol isolated from Metasequoia glyptostroboides
- Optimization of Extract Processing of Total Flavones of Metasequoia glyptostroboides Hu et Cheng with Uniform Design
- Articles : Phytochemical Constituents from Metasequoia glyptostroboids Leaves
- Terpenoids and Norlignans from Metasequoia glyptostroboides

Myricetin
- Structural Determinants of Phosphoinositide 3-Kinase Inhibition by Wortmannin, LY294002, Quercetin, Myricetin, and Staurosporine
- The anthocyanin reduced Tomato Mutant Demonstrates the Role of Flavonols in Tomato Lateral Root and Root Hair Development
- Hair wavingnatural product: Dillenia indica seed sap
- Myricetin suppresses UVB-induced wrinkle formationand MMP-9 expression by inhibiting Raf
- Antioxidant activity of myricetin p resence of taurine
- Active combinations, compositions and methods for enhancing hair growth
- Effects of Myricetin on the Bioavaiiability and Pharmacokinetics of Tamoxifen and its Main Metabolite, 4-Hydroxytamoxifen in Rats
- Chemopreventive effect of Myricetin, a natural occurring compound, on colonic chronic inflammation and inflammation-driven tumorigenesis in mice
- Effects of Myricetin, an Antioxidant, CYP2C9, 2D6 and P-gp Inhibitor in vitro, on the Pharmacokinetics of Carvedilol in Rats
- Anti-inflammatory activity of myricetin-3-O-β-D-glucuronide and related compounds
- Myricetin inhibits IL-1β-induced inflammatory mediators in SW982 human synovial sarcoma cells
- Protective effect of myricetin in dextran sulphate sodium-induced murine ulcerative colitis
- Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-κB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated RAW264.7 macrophages
- Ameliorative effect of myricetinon insulin resistance in mice fed a high-fat, high-sucrose diet
- Myricetin: A Dietary Molecule with Diverse Biological Activities
- Dual Action of Myricetin on Porphyromonas gingivalis and the Inflammatory Response of Host Cells: A Promising Therapeutic Molecule for Periodontal Diseases
- Antinociceptive and anti-inflammatory effects of myricetin3-O-β-galactoside isolated from Davilla elliptica: involvement of the nitrergic system
- Effect of Trimeric Myricetin Rhamnoside (TMR) in Carrageenan‐induced Inflammation and Caecal Ligation and Puncture‐induced Lung Oxidative Stress in Mice
- Myricetin suppresses LPS-induced MMP expression in human gingival fibroblasts and inhibits osteoclastogenesis by downregulating NFATc1 in RANKL-induced RAW 264.7 cells
- MKK4 is a novel target for the inhibition of tumor necrosis factor-α-induced vascular endothelial growth factor expression by myricetin
- Protective effects of apigenin and myricetin against cisplatin-induced nephrotoxicity in mice
- Quercetin and its principalmetabolites, but notmyricetin, opposelipopolysaccharide-inducedhyporesponsivenessofthe porcine isolatedcoronary artery
- Myricetin prevents titanium particle-induced osteolysis in vivo and inhibits RANKL-induced osteoclastogenesis in vitro
- Myricetin Protects Against Cytokine-Induced Cell Death in RIN-m5f β Cells
- Myricetin: biological activity related to human health
- Myricetin suppresses lipoteichoic acid‐induced interleukin‐1β and cyclooxygenase‐2 expression in human gingival fibroblasts
- Myricetin blocks lipoteichoic acid-induced COX-2 expression in human gingival fibroblasts
- Myricetin and quercetin are naturally occurring co-substrates of cyclooxygenases in vivo
- The dietary flavonoid myricetin regulates iron homeostasis by suppressing hepcidin expression
- Systems Pharmacology Dissection of the Protective Effect of Myricetin Against Acute Ischemia/Reperfusion-Induced Myocardial Injury in Isolated Rat Heart
- Research Article Myricetin Exerts Anti-osteoarthritic Effects in IL-1β Stimulated SW1353 Cells via Regulating Matrix Metalloproteinases and Modulating JNK
- Myricetin Down-Regulates Phorbol Ester-Induced Cyclooxygenase-2 Expression in Mouse Epidermal Cells by Blocking Activation of Nuclear Factor Kappa B
- Supplemental Naringenin Prevents Intestinal Barrier Defects and Inflammation in Colitic Mice
- Dietary naringenin supplementation attenuates experimental autoimmune encephalomyelitis by modulating autoimmuneinflammatory responses in mice
- Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice
- Naringenin is an inhibitor of T cell effector functions
- Naringenin inhibits osteoclastogenesis through modulation of helper T cells‐secreted IL‐4
- Regulation of Inflammatory Cytokinesin Lipopolysaccharide-Stimulated RAW 264.7 Murine Macrophage by 7-O-Methyl-naringenin
- Naringenin Suppresses NeuroinflammatoryResponses Through Inducing Suppressor of CytokineSignaling 3 Expression
- Naringenin has anti‐inflammatory properties in macrophage and ex vivo human whole‐blood models
- Naringenin Inhibits Superoxide Anion-Induced Inflammatory Pain: Role of Oxidative Stress, Cytokines,Nrf-2 and the NO−cGMP−PKG−KATPChannel Signaling Pathway
- Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-κB activity in a murine model of asthma
- Flavonoid Naringenin: A Potential Immunomodulator for Chlamydia trachomatis Inflammation
- Naringenin reducesinflammatory pain in mice
- Anti-inflammatoryrole of naringenin in rats with ethanol induced liver injury
- An Eco‐Friendly Enantioselective Access to (R)‐Naringenin as Inhibitor of Proinflammatory Cytokine Release
- The citrus flavanone naringenin inhibitsinflammatorysignalling in glial cells and protects against neuroinflammatory injury
- The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-κB activation
- Naringenin Ameliorates Acute Inflammation by Regulating Intracellular Cytokine Degradation
- Naringenin improves the healing process of thermally-induced skin damage in rats
- In vitroHair Growth Promoting Effects of Naringenin and Hesperetin on Human Dermal Papilla Cells and Keratinocytes
- Citrus flavanone naringenin enhances melanogenesisthrough the activation of Wnt/β-catenin signalling in mouse melanoma cells
- Anti-allergic Activity of Naringenin Chalcone from a Tomato Skin Extract
- Skin care compositions containing naringenin and/or quercetin and a retinoid
- Topical Formulation Containing Naringenin: Efficacy against Ultraviolet B Irradiation-Induced SkinInflammation and Oxidative Stress in Mice
- The inhibitory effect of naringenin on atopic dermatitis induced by DNFB in NC/Nga mice
- Naringenin ameliorates skin inflammation and accelerates phenotypic reprogramming from M1 to M2 macrophage polarization in atopic dermatitis NC/Nga mouse model.
- Effects of Naringenin on Inflammation in Complete Freund’s Adjuvant-Induced Arthritis by Regulating Bax/Bcl-2 Balance
- Naringenin prevents cholesterol-induced systemic inflammation, metabolic dysregulation, and atherosclerosis in Ldlr−/− mice
- Naringenin reduces cholesterol-induced hepatic inflammation in rats by modulating matrix metalloproteinases-2, 9 via inhibition of nuclear factor κB pathway
- Naringeninneutralises oxidative stress and nerve growth factor discrepancy in experimental diabetic neuropathy
- Protective Effect of Naringenin Against Lead-Induced Oxidative Stressin Rats
- The citrus flavonoids hesperetin and naringenin block the lipolytic actions of TNF-α in mouse adipocytes
- Naringenin inhibits TNF-α induced VSMC proliferation and migration via induction of HO-1
- TNF-α Blocker Effect of Naringenin-Loaded Sericin Microparticles that Are Potentially Useful in the Treatment of Psoriasis
- Anti-inflammatory effect of naringin and naringenin on TNF-α secretion in cultured cortical astrocytes after stimulation with LPS
- Naringenin promotes recovery from colonic damage through suppression of epithelial TNF-α production and induction of M2-type macrophages in colitic mice [2019]
- Naringenin ameliorates streptozotocin-induced diabetic rat renal impairment by downregulation of TGF-β1 and IL-1 via modulation of oxidative stress correlates with decreased apoptotic events
- Naringenin: an immunomodulator of Interleukin (IL)-6, IL-8, and Tumor Necrosis Factor secretion from Chlamydia trachomatis-infected macrophages and epithelial cells (110.28)
- INHIBITORY EFFECT OF NARINGENIN AND NARINGIN ON IL-6 AND IL-8 RELEASE: 60

- Protective Effect of Naringenin Against Lead-Induced Oxidative Stress in Rats
- Therapeutic Potential of Naringin for Intervertebral Disc Degeneration: Involvement of Autophagy Against Oxidative Stress-Induced Apoptosis in Nucleus Pulposus Cells
- Mice pancreatic islets protection from oxidative stress induced by single-walled carbon nanotubes through naringin
- Amelioration of autoimmune arthritis by naringinthrough modulation of T regulatory cells and Th1/Th2 cytokines
- Naringin Attenuates the Development of Carrageenan-Induced Acute Lung Inflammation Through Inhibition of NF-κb, STAT3 and Pro-Inflammatory Mediators and Enhancement of IκBα and Anti-Inflammatory Cytokines
- Naringin protects ultraviolet B-induced skin damageby regulating p38 MAPK signal pathway
- Naringin, a flavanone glycoside, promotes angiogenesis and inhibits endothelial apoptosis through modulation ofinflammatory and growth factor expression in diabetic foot ulcer in rats
- Naringin improves random skin flap survival in rats
- Naringin Protects against Kainic Acid-Induced Status Epilepticus in Rats: Evidence for an Antioxidant, Anti-inflammatory and Neuroprotective Intervention
- A novel ultradeformable liposomes of Naringin for anti-inflammatory therapy
- Study on the antiinflammation and analgesia of naringin
- Analysis of ionizing radiation‐induced DNA damage and repair in three‐dimensional human skin model system
- Naringin ameliorates pentylenetetrazol-induced seizures and associated oxidative stress, inflammation, and cognitive impairment in rats: Possible mechanisms of neuroprotection
- Naringin prevents ultraviolet‐B radiation‐induced oxidative damage and inflammation through activation of peroxisome proliferator‐activated receptor γ in mouse embryonic fibroblast (NIH‐3T3) cells
- NARINGIN REVEALS AMELIORATIVE PROPERTY OVER ELEVATED OXIDATIVE STRESS LEVELS IN ANIMAL MODELS
- Wound healing potential of naringin ointment formulation via regulating the expression of inflammatory, apoptotic and growth mediators in experimental rats
- Naringin Inhibits Tumor Growth and Reduces Interleukin-6 and Tumor Necrosis Factor α Levels in Rats with Walker 256 Carcinosarcoma
- Anti-Inflammatory Effects of Naringin in Chronic Pulmonary Neutrophilic Inflammation in Cigarette Smoke-Exposed Rats
- Therapeutic effects of silymarin and naringin on methotrexate‐induced nephrotoxicity in rats: Biochemical evaluation of anti‐inflammatory,antiapoptotic, and antiautophagic properties
- Naringin Protects Ovalbumin-Induced Airway Inflammation in a Mouse Model of Asthma
- Naringin attenuates diabetic retinopathy by inhibiting inflammation, oxidative stress and NF-κB activation in vivo and in vitro
- Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage
- NARINGIN AND RUTIN PREVENT D-GALACTOSAMINE-INDUCED HEPATIC INJURY IN RATS VIA ATTENUATION OF THE INFLAMMATORY CASCADE AND OXIDATIVE STRESS
- Effect of naringin enzymatic hydrolysis towards naringenin on the anti-inflammatory activity of both compounds
- Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway
- Naringin ameliorates gentamicin-induced nephrotoxicity and associated mitochondrial dysfunction, apoptosis and inflammation in rats: Possible mechanism of nephroprotection
- Anti-inflammatory activity of naringin and the biosynthesised naringenin by naringinase immobilized in microstructured materials in a model of DSS-induced colitis in mice
- Naringin attenuates enhanced cough, airway hyperresponsiveness and airway inflammation in a guinea pig model of chronic bronchitis induced by cigarette smoke
- Protective role of naringin against cisplatin induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing ROS-mediated NF-κB and P53 signaling pathways
- Naringin Alleviates Diabetic Kidney Disease through Inhibiting Oxidative Stress and Inflammatory Reaction
- Naringin ameliorates sodium arsenite-induced renal and hepatic toxicity in rats: decisive role of KIM-1, Caspase-3, TGF-β, and TNF-α
- Naringin rescued the TNF-α-induced inhibition of osteogenesis of bone marrow-derived mesenchymal stem cells by depressing the activation of NF-кB signaling pathway
- Naringin Decreases TNF-α and HMGB1 Release from LPS-Stimulated Macrophages and Improves Survival in a CLP-Induced Sepsis Mice
- Naringin Inhibits TNF-α Induced Oxidative Stress and Inflammatory Response in HUVECs via Nox4/NF-Κ B and PI3K/Akt Pathways
- Inhibition of TNF-α/IFN-γ induced RANTES expression in HaCaT cell by naringin

- Oleuropein an Olive Oil Compound in Acute and Chronic Inflammation Models: Facts and Perspectives
- Topical Application of Oleuropein Induces Anagen Hair Growth in Telogen Mouse Skin
- Pre-Initiation Effect of Oleuropein towards Apoptotic and Oxidative Stress Levels on the Early Development of Two-Stage Skin Carcinogenesis
- Olive Leaf Extract and Its Main Component OleuropeinPrevent Chronic Ultraviolet B Radiation-Induced Skin Damage and Carcinogenesis in Hairless Mice
- Evaluation of Effect of Oleuropein on Skin Wound Healing in Aged Male Balb/c Mice
- Therapeutic Effects of Oleuropein on Wounded Skinin Young Male Balb/c Mice
- Oleuropein Decreases Cyclooxygenase-2 and Interleukin-17 Expression and AttenuatesInflammatory Damage in Colonic Samples from Ulcerative Colitis Patients
- Oleuropein inhibited Th17 response and reduced intestinal IL-17 and IFN-γ release in dextran sulfate sodium (DSS)-induced acute colitis in C57BL/6 mice
- Oleuropein alleviates malathion-induced oxidative stress and DNA damage in rats
- OC.14.4: Oleuropein Decreases Interleukin (IL)-17 and Attenuates Inflammatory Damage in Colonic Mucosa from Ulcerative Colitis Patients
- Oleuropein Suppresses LPS-Induced Inflammatory Responses in RAW 264.7 Cell and Zebrafish
- Anti-Inflammatory Effect of Oleuropein in Experimental Rat Spinal Cord Trauma
- Olive oil and its main phenolic micronutrient (oleuropein) prevent inflammation-induced bone loss in the ovariectomised rat
- Effects of oleuropein on serum inflammatory cytokines and histopathological changes in rats with pancreatitis.
- Oleuropein down-regulated IL-1β-inducedinflammation and oxidative stress in human synovial fibroblast cell line SW982
- Effect of oleuropein on cognitive deficits and changes in hippocampal brain-derived neurotrophic factor and cytokine expression in a rat model of post-traumatic stress disorder
- Dose–response study of effect of oleuropein, an olive oil polyphenol, in an ovariectomy/inflammationexperimental model of bone loss in the rat
- Anti-inflammatory effect of oleuropein on microglia through regulation of Drp1-dependent mitochondrial fission
- Oleuropein inhibits the IL-1β-induced expression ofinflammatory mediators by suppressing the activation of NF-κB and MAPKs in human osteoarthritis chondrocytes
- Oleuropein prevents oxidative myocardial injury induced by ischemia and reperfusion
- Antioxidant Status and Anti-inflammatory Effects ofOleuropein in Streptozotocin-induced Diabetic Nephropathy in Rats
- Oleuropein Curtails Pulmonary Inflammation and Tissue Destruction in Models of Experimental Asthma and Emphysema
- The leishmanicidal activity of oleuropein is selectively regulated through inflammation– and oxidative stress-related genes
- Acute doxorubicin cardiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress
- Association of genotypes affecting the expression of interleukin‐1β or interleukin‐1 receptor antagonist with osteoarthritis
- Oleuropein protects against ethanol-induced oxidative stress and modulates sperm quality in the rat testis
- Oleuropein improves mitochondrial function to attenuate oxidative stress by activating the Nrf2 pathway in the hypothalamic paraventricular nucleus of spontaneously hypertensive rats
- Antioxidant effects of oleuropein versus oxidative stress induced by ethanol in the rat intestine
- Oleuropein ameliorates arsenic induced oxidative stress in mice
- Effect of oleuropein against chemotherapy drug-induced histological changes, oxidative stress, and DNA damages in rat kidney injury
- Paeoniflorin inhibitsinflammatoryresponses in mice with allergic contact dermatitis by regulating the balance between inflammatory and anti-inflammatorycytokines
- Paeoniflorin protects against lipopolysaccharide-induced acute lung injury in mice by alleviatinginflammatory cell infiltration and microvascular permeability
- Paeoniflorin Inhibits Systemic Inflammation and Improves Survival in Experimental Sepsis
- Paeoniflorinattenuates allergic inflammation in asthmatic mice
- Paeoniflorin inhibits skin lesions in imiquimod-induced psoriasis-like mice by downregulating inflammation
- Immunoregulatory Effects of PaeoniflorinExerts Anti-asthmatic Effects via Modulation of the Th1/Th2Equilibrium
- Oral administration of paeoniflorin attenuates allergic contact dermatitis by inhibiting dendritic cell migration and Th1 and Th17 differentiation in a mouse model
- Paeoniflorin suppressesinflammatory response in imiquimod-induced psoriasis-like mice and peripheral blood mononuclear cells (PBMCs) from psoriasis patients
- Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities
- Paeoniflorin inhibits function and down-regulates HLA-DR and CD80 expression of human peripheral blood monocytes stimulated by RhIL-1β
- Paeoniflorin down-regulates ATP-induced inflammatory cytokine production and P2X7R expression on peripheral blood mononuclear cells from patients with primary Sjögren’s syndrome
- Paeoniflorin Protects against Ischemia-Induced Brain Damages in Rats via Inhibiting MAPKs/NF-κB-Mediated Inflammatory Responses
- PaeoniflorinAtttenuates Amyloidogenesis and the Inflammatory Responses in a Transgenic Mouse Model of Alzheimer’s Disease
- Paeoniflorin Ameliorates Experimental Autoimmune Encephalomyelitis via Inhibition of Dendritic Cell Function and Th17 Cell Differentiation
- Paeoniflorin suppresses IL-6/Stat3 pathway via upregulation of Socs3 in dendritic cells in response to 1-chloro-2,4-dinitrobenze
- Paeoniflorin diminishes ConA-induced IL-8 production in primary human hepatic sinusoidal endothelial cells in the involvement of ERK1/2 and Akt phosphorylation
- Paeoniflorin augments systemic Candida albicans infection through inhibiting Th1 and Th17 cellexpression in a mouse model
- Paeoniflorin reduces neomycin-induced ototoxicity in hair cellsby suppression of reactive oxygen species generation and extracellularly regulated kinase signalization
- The Anti-Inflammatory Effect of Paeoniflorin on Cerebral Infarction Induced by Ischemia-Reperfusion Injury in Sprague-Dawley Rats
- Paeoniflorinprevents TLR2/4-mediated inflammation in type 2 diabetic nephropathy
- Partially purified paeoniflorin exerts protective effects on UV-induced DNA damage and reduces facial wrinkles in human skin.
- The skin‐depigmenting potential of Paeonia lactiflora root extract and paeoniflorin: in vitro evaluation using reconstructed pigmented human epidermis
- BIOLOGICALLY ACTIVE PRINCIPLES OF CRUDE DRUGS. II. ANTI-ALLERGIC PRINCIPLES IN “SHOSEIRYU-TO” ANTI-INFLAMMATORYPROPERTIES OF PAEONIFLORIN AND ITS DERIVATIVES
- Paeoniflorin improves survival in LPS-challenged mice through the suppression of TNF-α and IL-1βrelease and augmentation of IL-10 production
- Effects of recombinant human interleukin-1β on functions of peripheral blood mononuclear cells from healthy adults and the role of paeoniflorin
- Paeoniflorin attenuates amyloid-beta peptide-induced neurotoxicity by ameliorating oxidative stress and regulating the NGF-mediated signaling in rats
- Protective effect of paeoniflorinagainst oxidative stress in human retinal pigment epithelium in vitro
- Paeoniflorin protects against ANIT-induced cholestasis by ameliorating oxidative stress in rats
- Paeoniflorin ameliorates rheumatoid arthritis in rat models through oxidative stress, inflammation and cyclooxygenase 2
- Inhibitory effect of paeoniflorin on methylglyoxal-mediated oxidative stress in osteoblastic MC3T3-E1 cells
- Effects of Paeoniflorin on Serum Levels of TNF-α、IL-6、IL-10 in Rat Hepatic Fibrosis Model
- Study of Blood Nourishing Effects of Albiflorin and Paeoniflorin on Blood Deficiency Mouse Model Induced by Irradiation of and Effect on Levels of IL-3,GM-CSF,IL-6 and TNF-α
- Pharmacological effects of Paeoniflorin and Albiflorin on IL-3, GM-CSF, IL-6 and TNF-α in the rats of syndrome of stagnation of liver qi and blood deficiency
- Paeoniflorin protects cells from GalN/TNF-α-induced apoptosis via ER stress and mitochondria-dependent pathways in human L02 hepatocytes
- [Comparative study on effects of blood enriching on mouse model of blood deficiency syndrome induced by cyclophosphamide of albiflorin, paeoniflorin on levels of GM-CSF, IL-3 and TNF-α].
- Paeoniflorin Antagonizes TNF-α-Induced L929 Fibroblastoma Cells Apoptosis by Inhibiting NF-κBp65 Activation
- Paeoniflorin alleviates TNF-α induced intestinal barrier dysfunction by inhibition of MLCK
- [Inhibition of Paeoniflorin on TNF-α-induced TNF-α Receptor Type I /Nuclear Factor-κB Signal Transduction in Endothelial Cells].
- Influence of Paeoniflorin on Contents of Interleukin – 1β,Interleukin – 4 and TNF – α in Rats with Ulcerative Colitis
- Paeoniflorin induces the apoptosis of human gastric carcinoma cells by inhibiting activation of NF-κB
- Neuroprotection by Paeoniflorin against Nuclear Factor Kappa B-Induced Neuroinflammation on Spinal Cord Injury

Perilla frutescens
- A WD-repeat-containing putative regulatory proteinin anthocyanin biosynthesis in Perilla frutescens
- Plant regeneration in vitro directly from cotyledon and hypocotyl explants of Perilla frutescens and their morphological aspects
- STUDIES ON THE MORPHOLOGY,STRUCTURE AND DEVELOPMENT OF THE GLANDULAR HAIRS IN PERILLA FRUTESCENS (L. )BRITTON
- MORPHOGENESIS OF GLANDULAR HAIRS ON THE LEAVES OF PERILLA FRUTESCENS (L. ) BRITTON
- The promotion of hair regrowth by topical application of a Perilla frutescens extractthrough increased cell viability and antagonism of testosterone and dihydrotestosterone
- Active Volatile Constituents in Perilla frutescensEssential Oils and Improvement of Antimicrobial and Anti-Inflammatory Bioactivity by Fractionation
- Identification and quantification of essential oil content and composition, total polyphenols and antioxidant capacityof Perilla frutescens (L.) Britt
- Purification and identification of two novel antioxidantpeptides from perilla (Perilla frutescens L. Britton) seed protein hydrolysates
- In Vitro and in Vivo Effects of Macrophage-Stimulatory Polysaccharide from Leaves of Perilla frutescensvar. crispa
- Effect of Perilla frutescens var. acuta Kudo and rosmarinic acid on allergic inflammatory reactions
- Biological evaluation of isoegomaketone isolated from Perilla frutescens and its synthetic derivatives as anti-inflammatory agents
- Anti-inflammatory Effect of Perilla frutescens (L.) Britton var. frutescens Extract in LPS-stimulated RAW 264.7 Macrophages
- Anti‑inflammatory effects of Perilla frutescensleaf extract on lipopolysaccharide‑stimulated RAW264.7 cells
- Inhibition of Proinflammatory Cytokine Generation in Lung Inflammation by the Leaves of Perilla frutescensand Its Constituents
- Perilla frutescens extractameliorates DSS-induced colitis by suppressing proinflammatory cytokines and inducing anti-inflammatory cytokines
- Phytochemical profiles and in vitro anti-inflammatory properties of Perilla frutescens cv. Chookyoupjaso mutants induced by mutagenesis with γ-ray
- Perilla frutescens Leaf ExtractInhibits Mite Major Allergen Der p 2-induced Gene Expression of Pro-Allergic and Pro-Inflammatory Cytokines in Human Bronchial Epithelial Cell BEAS-2B
- Anti-inflammatory constituents from Perilla frutescens on lipopolysaccharide-stimulated RAW264.7 cells
- Perilla frutescens Extracts Protects against Dextran Sulfate Sodium-Induced Murine Colitis: NF-κB, STAT3, and Nrf2 as Putative Targets
- Comparison of anti-inflammatory activityof extracts with supercritical carbon dioxide from radiation mutant perilla frutescens(L.) Britton and wild-type
- Comparison of the Anti-Inflammatory Activities of Supercritical Carbon Dioxide versus Ethanol Extracts from Leaves of Perilla frutescens Britt. Radiation Mutant
- Anti-inflammatory Activity of Perilla frutescens Britton Seed in RAW 264.7 Macrophages and an Ulcerative Colitis Mouse Model
- Triterpene Acids from the Leaves of Perilla frutescens and Their Anti-inflammatory and Antitumor-promoting Effects
- Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model
- β-secretase (BACE1) inhibitors from Perilla frutescensvar. acuta
- Protective effect of aqueous extract of Perilla frutescenson tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats
- The hepatoprotection of caffeic acid and rosmarinic acid, major compounds of Perilla frutescens, against t-BHP-induced oxidative liver damage
- Ethanol Extract of Perilla frutescens Suppresses Allergen-Specific Th2 Responsesand Alleviates Airway Inflammation and Hyperreactivity in Ovalbumin-Sensitized Murine Model of Asthma
- Anti-inflammatory effects of Perilla frutescens in activated human neutrophils through two independent pathways: Src family kinases and Calcium
- Hypolipidemic and antioxidant effects of total flavonoids of Perilla Frutescens leaves in hyperlipidemia rats induced by high-fat diet
- Perilla Frutescens targets intestinal permeability
In vitro study on TNF-α stress-induced barrier dysfunction in intestinal epithelial cells
Pinocembrin
- Pinocembrin attenuates gentamicin-induced nephrotoxicityin rats
- Pinocembrin attenuates blood–brain barrier injury induced by global cerebral ischemia–reperfusion in rats
- The characteristics of therapeutic effect of pinocembrin in transient global brain ischemia/reperfusion rats
- Use of racemates of pinocembrin in preparing medicaments for treating stroke
- In vitro and in vivo protection provided by pinocembrinagainst lipopolysaccharide-induced inflammatory responses
- Pinocembrin attenuates hippocampal inflammation, oxidative perturbations and apoptosis in a rat model of global cerebral ischemia reperfusion
- Pinocembrin protects hemorrhagic brain primarily by inhibiting toll-like receptor 4 and reducing M1 phenotype microglia
- Protection of mice against lipopolysaccharide-induced endotoxic shock by pinocembrin is correlated with regulation of cytokine secretion
- Pinocembrin Protects Human Brain Microvascular Endothelial Cells against Fibrillar Amyloid- β1−40 Injury by Suppressing the MAPK/NF- κ B Inflammatory Pathways
- Pinocembrin Inhibits Matrix MetalloproteinaseExpression in Chondrocytes
- Pinocembrin attenuates lipopolysaccharide–induced inflammatory responses in Labeo rohita macrophages via the suppression of the NF-κB signalling pathway
- Pinocembrin, a novel histidine decarboxylase inhibitor with anti-allergic potential in in vitro
- Pinocembrin inhibits lipopolysaccharide-induced inflammatory mediators production in BV2 microglial cells through suppression of PI3K/Akt/NF-κB pathway
- Pinocembrin– flavonoid component of domestic propolis with delaying effect of the development of Alzheimer’s disease
- Pinocembrin improves cognition and protects the neurovascular unit in Alzheimer related deficits
- THERAPEUTIC EFFECTS OF PINOCEMBRIN ON INDOMETHACIN-INDUCED GASTRIC ULCER MODEL IN RATS
- Pinocembrin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress induced apoptosis
- Protections of pinocembrin on brain mitochondriacontribute to cognitive improvement in chronic cerebral hypoperfused rats
- Pinocembrin Attenuates 6-OHDA-induced Neuronal CellDeath Through Nrf2/ARE Pathway in SH-SY5Y Cells
- Inhibition of beta-amyloid-induced neurotoxicity by pinocembrin through Nrf2/HO-1 pathway in SH-SY5Y cells
- Pinocembrin protects rat brain against oxidation and apoptosis induced by ischemia–reperfusion both in vivo and in vitro
- Pinocembrin Protects SH-SY5Y Cells Against MPP+-Induced Neurotoxicity Through the Mitochondrial Apoptotic Pathway
- Pinocembrin reduces cardiac arrhythmiaand infarct size in rats subjected to acute myocardial ischemia/reperfusion
- Pinocembrin protects against β-amyloid-induced toxicityin neurons through inhibiting receptor for advanced glycation end products (RAGE)-independent signaling pathways and regulating mitochondrion-mediated apoptosis
- Pinocembrin attenuates MPP+-induced neurotoxicity by the induction of heme oxygenase-1 through ERK1/2 pathway
- Galangin and Pinocembrin from Propolis Ameliorate Insulin Resistancein HepG2 Cells via Regulating Akt/mTOR Signaling
- Neuroprotective effects of pinocembrin on ischemia/reperfusion-induced brain injury by inhibiting autophagy
- Pinocembrin induces ER stress mediated apoptosis and suppresses autophagy in melanoma cells
- Pinocembrin Protects the Brain against Ischemia-Reperfusion Injury and Reverses the Autophagy Dysfunction in the Penumbra Area
- Antiproliferative and apoptotic effects of pinocembrin in human prostate cancer cells

- Leishmanicidal Activity of Piper nigrum Bioactive Fractions is Interceded via Apoptosis In Vitro and Substantiated by Th1 Immunostimulatory Potential In Vivo
- Piper nigrum extractameliorated allergic inflammation through inhibiting Th2/Th17 responsesand mast cells activation
- Anti-inflammatory activityand chemical composition of dichloromethane extract from Piper nigrum and P. longum on permanent focal cerebral ischemia injury in rats
- Piperine inhibits cytokine production by human peripheral blood mononuclear cells
- Piperine is a potent inhibitor of nuclear factor-κB (NF-κB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F-10 melanoma cells
- Alkaloids from Piper nigrum Exhibit Antiinflammatory Activity via Activating the Nrf2/HO1 Pathway
- Piperine inhibits IL-β induced expression of inflammatory mediators in human osteoarthritis chondrocyte
- Piper nigrum Fruit Extract Prevents TMA-Induced Allergic Contact Dermatitis by Regulating Th2 CytokineProduction
- Melanogenesis Stimulation in Murine B16 Melanoma Cells by Piper nigrum Leaf Extract and Its Lignan Constituents
- Alkamides from Piper longum and Piper nigrum as Inhibitors of IL-6 action
- Antioxidant efficacy of black pepper (Piper nigrum L.)and piperine in rats with high fat diet induced oxidative stress
- Methanolic Extract of Piper nigrum Fruits Improves Memory Impairment by Decreasing Brain Oxidative Stress in Amyloid Beta(1–42) Rat Model of Alzheimer’s Disease
- Piper nigrum ethanolic extract rich in piperamides causes ROS overproduction, oxidative damagein DNA leading to cell cycle arrest and apoptosis in cancer cells
- γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress
- Antioxidant, Anti-inflammatory and Antinociceptive Properties of Black Pepper Essential Oil (Piper nigrum Linn)
- Polyphenolic extract of Piper nigrum inhibitssodium oxalate induced oxidative stress in rat kidney
- Analgesic and anti-inflammatoryactivities of Piper nigrum L.
- Piperine and quercetin enhances antioxidant and hepatoprotective effect of curcumin in paracetamol induced oxidative stress
- Piperine modulation of carcinogen induced oxidative stress in intestinal mucosa
- Modulation of cadmium induced alterations in murine thymocytes by piperine: Oxidative stress,apoptosis, phenotyping and blastogenesis
- Piperine ameliorates oxidative stress, inflammationand histological outcome in collagen induced arthritis
- Piperine potentiates the protective effects of curcumin against chronic unpredictable stress-induced cognitive impairment and oxidative damage in mice
- Attenuation of beryllium induced hepatorenal dysfunction and oxidative stress in rodents by combined effect of gallic acid and piperine
- Quercetin along with piperine prevents cognitive dysfunction, oxidative stress and neuro-inflammation associated with mouse model of chronic unpredictable stress
- The use of nitroxide radical-containing nanoparticles coupled with piperine to protect neuroblastoma SH-SY5Y cells from Aβ-induced oxidative stress
- Piperineinhibits eosinophil infiltration and airway hyperresponsiveness by suppressing T cell activity and Th2 cytokine production in the ovalbumin‐induced asthma model
- Caloric restriction favorably impacts metabolic and immune/inflammatory profiles in obese mice but curcumin/piperine consumption adds no further benefit
- Piperinesuppresses cerebral ischemia–reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-κB in middle cerebral artery occlusion rat model
- Anti-apoptotic and Anti-inflammatory effect of Piperine on 6-OHDA induced Parkinson’s Rat model
- Anti‐Inflammatory Effects of Capsaicin and Piperine on Helicobacter pylori‐Induced Chronic Gastritis in Mongolian Gerbils
- Inhibitory Effects of Black Pepper (Piper Nigrum) Extracts and Compounds on Human Tumor Cell Proliferation, Cyclooxygenase Enzymes, Lipid Peroxidation and Nuclear Transcription Factor-kappa-B

- Inhibition of HIV-1 enzymes, antioxidant and anti-inflammatory activities of Plectranthus barbatus
- Protection against hepatic oxidative damage induced by iron by the aqueous extract of Plectranthus barbatus
- In Vitro Skin Diffusion Study of Pure Forskolin versus a Forskolin-Containing Plectranthus barbatus Root Extract

Polyporus umbellatus
- Studies of the Active Substances in Herbs Used for Hair Treatment. II.Isolation of Hair Regrowth Substances,Acetosyringone and Polyporusterone A and B, from Polyporus umbellatus Fries
- Studies on Active Substances in Herbs Used for Hair Treatment.I. Effects of Herb Extracts on Hair Growth and Isolation of an Active Substance from Polyporus umbellatus F.
- Traditional uses, phytochemistry, pharmacology, pharmacokinetics and quality control of Polyporus umbellatus (Pers.) Fries: A review
- Studies of Active Substances in Herbs Used for Hair Treatment. IV. The Structure of the Hair RegrowthSubstance, Polyporusterone A, from Polyporus umbellatus F.
- Diuretic activity and kidney medulla AQP1, AQP2, AQP3, V2R expression of the aqueous extract of sclerotia of Polyporus umbellatusFRIES in normal rats
- Inhibitory Effects of Triterpenes Isolated from Chuling (Polyporus umbellatus FRIES) on Free Radical-Induced Lysis of Red Blood Cells
- Polyporus umbellatus, an Edible-Medicinal Cultivated Mushroom with Multiple Developed Health-Care Products as Food, Medicine and Cosmetics: A Review
- Fungal species residing in the sclerotia of Polyporus umbellatus
- Renoprotective effect and mechanism of polysaccharide from Polyporus umbellatussclerotia on renal fibrosis
- TLR4-mediated activation of macrophages by the polysaccharide fraction from Polyporus umbellatus(pers.) Fries
- Polysaccharide purified from Polyporus umbellatus (Per) Fr induces the activation and maturation of murine bone-derived dendritic cells via toll-like receptor 4
- Polyporus umbellatus, an Edible-Medicinal Cultivated Mushroom with Multiple Developed Health-Care Products as Food,Medicine and Cosmetics: A Review
- Nutritional factors determining sclerotial formation of Polyporus umbellatus
- Determination of optimal carbon source and pH value for sclerotial formation of Polyporus umbellatus under artificial conditions
- Growth Promoting Effects of Water Extract of Armillaria mellea Rhizomorph on Myceliaof Polyporus umbellatus
- Nox Gene Expression and Cytochemical Localization of Hydrogen Peroxide in Polyporus umbellatusSclerotial Formation
- Induction of apoptosis in MCF‑7 human breast cancer cells by Khz (fusion of Ganoderma lucidum and Polyporus umbellatus mycelium)
- New anti-inflammatory ergostane-type ecdysteroids from the sclerotium of Polyporus umbellatus
- Cytotoxic Steroids from Polyporus umbellatus
- Polyporus umbellatuspolysaccharides ameliorates carbon tetrachloride-induced hepatic injury in mice
- Structure and chain conformation of a neutral polysaccharide from sclerotia of Polyporus umbellatus
- Bioactivity-directed isolation, identification of diuretic compounds from Polyporus umbellatus
- Ergosta‐4,6,8(14),22‐tetraen‐3‐one isolated from Polyporus umbellatusprevents early renal injury in aristolochic acid‐induced nephropathy rats
- Polyporus umbellatus inhibited tumor cell proliferation and promoted tumor cell apoptosis by down-regulating AKT in breast cancer
- Genetic Diversity and Evolution of Chinese Traditional Medicinal Fungus Polyporus umbellatus (Polyporales, Basidiomycota)
- Khz (Fusion Product of Ganoderma lucidum and Polyporus umbellatus Mycelia)Induces Apoptosis in Human Colon Carcinoma HCT116 Cells, Accompanied by an Increase in Reactive Oxygen Species, Activation of Caspase 3, and Increased Intracellular Ca2+
- Simultaneous determination of eight major steroids from Polyporus umbellatus by high‐performance liquidchromatography coupled with mass spectrometry detections
- DETERMINATION OF MUSHROOM MONOSACCHARIDES BY LIQUID CHROMATOGRAPHY MASS-SPECTROMETRY (LCMS) AND MACROPHAGE ACTIVATION BY POLYSACCHARIDES FROM Polyporus umbellatus, Fuscoporia obliqua, Cordyceps militaris AND Pleurotus ostreatus
- GPP (Composition of Ganoderma Lucidum Poly-saccharides and Polyporus Umbellatus Poly-saccharides) Enhances Innate Immune Function in Mice
- Transcriptome analysis of genes involved in defence response in Polyporus umbellatus with Armillaria mellea infection
- The Study of Polyporus Umbellatus Polysaccharide Effecting on Expressions of Mouse HepA Tumor Cell P16? Rb Gene and TNF-α [J]
- Study of Effect of Polyporus Umbellatus and Alisma Orientalis on TGF-β_1 and TNF-α in Serum of Pulmonary Fibrosis Rat Model

- Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: potential implication of the gut microbiota
- Polyphenol-rich pomegranate fruitextract (POMx) suppresses PMACI-induced expression of pro-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-κB in human KU812 cells
- Effects of pomegranate juice in circulating parameters, cytokines, and oxidative stress markers in endurance-based athletes: A randomized controlled trial
- Potential Anti-Inflammatory Effects of the Hydrophilic Fraction of Pomegranate (Punica granatum L.) Seed Oil on Breast Cancer Cell Lines
- Effects of pomegranate juice consumption oninflammatory markers in patients with type 2 diabetes: A randomized, placebo-controlled trial
- Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats
- The Effects of Pomegranate Juice on ProinflammatoryCytokines and Physical Function in Patients With Knee Osteoarthritis
- Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in Rheumatoid Arthritis patients
- Potential Effects of Pomegranate on Lipid Peroxidation and Pro-inflammatory Changes in Daunorubicin-induced Cardiotoxicity in Rats
- Anti-Inflammatory Mechanism Involved in Pomegranate-Mediated Prevention of Breast Cancer: the Role of NF-κB and Nrf2 Signaling Pathways
- Polyphenolics from Mango (Mangifera indica L.) and Pomegranate (Punica granatum L.) Suppress Inflammation in in vivo and in vitro Models for Colitis
- Does Short Term Usage of Fresh Pomegranate Juice (FPJ) Protect Cochlear Hair Cells after Cisplatin-Based Chemo-Irradiation?
- Hair-dyeing by using Pomegranate Hull Extract
- Pomegranate seed oil nanoemulsions improve the photostability and in vivo antinociceptive effect of a non-steroidal anti-inflammatory drug
- Anthocyanin‐ and hydrolyzable tannin‐rich pomegranate fruit extract modulates MAPK and NF‐κB pathways and inhibits skin tumorigenesis in CD‐1 mice
- Protective effect of pomegranate‐derived products on UVB‐mediated damage in human reconstituted skin
- Protective Effects of Standardized Pomegranate (Punica granatum L.) Polyphenolic Extract in Ultraviolet-Irradiated Human Skin Fibroblasts
- Phenolic Compounds of Pomegranate Byproducts (Outer Skin, Mesocarp, Divider Membrane) and Their Antioxidant Activities
- Pomegranate Fruit Extract Inhibits UVB‐induced Inflammation and Proliferation by Modulating NF‐κB and MAPK Signaling Pathways in Mouse Skin
- Thermal characteristics of a water soluble extract obtained from pomegranate skin: Developing a state diagram for determining stability
- Synergistic growth inhibition of mouse skin tumors by pomegranatefruit extract and diallyl sulfide: Evidence for inhibition of activated MAPKs/NF-κB and reduced cell proliferation
- Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E–deficient mice
- Beneficial effects of pomegranate juice on oxidation-sensitive genes and endothelial nitric oxide synthase activity at sites of perturbed shear stress
- Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages
- Pomegranate reverses methotrexate-induced oxidative stress and apoptosis in hepatocytes by modulating Nrf2-NF-κB pathways
- PomegranateByproduct Administration to Apolipoprotein E-Deficient Mice Attenuates Atherosclerosis Development as a Result of Decreased Macrophage Oxidative Stress and Reduced Cellular Uptake of Oxidized Low-Density Lipoprotein
- One year of pomegranate juice intake decreases oxidative stress, inflammation,and incidence of infections in hemodialysis patients: A randomized placebo-controlled trial
- Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson’s disease
- Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts
- Effects of a Pomegranate Fruit Extract rich in punicalagin on oxidation-sensitive genes and eNOS activity at sites of perturbed shear stress and atherogenesis
- Effect of pomegranate seed oil on serum TNF-α level in dyslipidemic patients
- Pomegranate extract prevents skeletal muscle of mice against wasting induced by acute TNF‐α injection
- The effects of pomegranate seed oil supplementation on the PPAR gamma and GLUT4 genes expression and the serum levels of IL-6, TNF-α and hs-CRP inflammatory factors and Glycemic indexes in obese type 2 diabetic patients: randomized controlled clinical trial
- A Polyphenol‐rich Pomegranate Fruit Extract Suppresses NF‐κB and IL‐6 Expression by Blocking the Activation of IKKβ and NIK in Primary Human Chondrocytes
- THE EFFECT OF SIX WEEKS AEROBIC EXERCISE AND POMEGRANATE JUICE CONSUMPTION ON IL-6 IN WOMEN WHIT TYPE 2 DIABETIC
- Pomegranate Fruit Extract Modulates UV‐B–mediated Phosphorylation of Mitogen‐activated Protein Kinases and Activation of Nuclear Factor Kappa B in Normal Human Epidermal Keratinocytes
- Pomegranate fruit extract modulates UVB-induced activation of nuclear factor kappa B and phosphorylation of mitogen activated protein kinases in normal human epidermal keratinocytes
- 137: Evaluation of Pomegranate Polyphenol-Mediated Suppression of Nuclear Factor Kappa b (NF-KB) Activity in Prostate Cancer Cell Lines
- Oral administration of apple condensed tannins delays rheumatoid arthritis development in mice via downregulation of T helper 17 (Th17) cell responses
- Oligomeric Procyanidins Interfere with Glycolysis of Activated T Cells. A Novel Mechanism for Inhibition of T Cell Function
- Grape-seed procyanidinsprevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet
- Cocoa Procyanidins and Human CytokineTranscription and Secretion
- Effect of Cocoa Procyanidins on the Secretion of Interleukin-4 in Peripheral Blood Mononuclear Cells
- The in vitro evaluation of isolated procyanidinsas modulators of cytokine-induced eotaxin production in human alveolar epithelial cells
- Oral Administration of Apple Procyanidins Ameliorates Insulin Resistance via Suppression of Pro-Inflammatory Cytokine Expression in Liver of Diabetic ob/ob Mice
- Anti-Inflammatory Procyanidins and Triterpenes in 109 Apple Varieties
- Dietary Grape-Seed Procyanidins Decreased Postweaning Diarrhea by Modulating Intestinal Permeability and Suppressing Oxidative Stress in Rats
- Grape-Seed Procyanidins Act as AntiinflammatoryAgents in Endotoxin-Stimulated RAW 264.7 Macrophages by Inhibiting NFkB Signaling Pathway
- Effects of Grape Seed Procyanidins on Cytokines,Nitric Oxide Synthase and Blood-Brain Barrier After Cerebral Ischemia-Reperfusion in Mice
- Procyanidin B2 gallates inhibit IFN-γ and IL-17 production in T cells by suppressing T-bet and RORγt expression
- Multiple Cytokine Inhibition through Specific Procyanidin–EnrichedPlant Extracts: Implicationsfor the Treatment of Psoriasis, Eczema and Dermatitis
- Contribution of transcript stability to a conserved procyanidin-inducedcytokine response in γδ T cells
- Chemical Characterization of a Procyanidin-Rich Extract from Sorghum Bran and Its Effect on Oxidative Stress and Tumor Inhibition in Vivo
- Procyanidin B2 Protects Neurons from Oxidative, Nitrosative, and Excitotoxic Stress
- Antigenotoxic Effect of Grape Seed Procyanidin Extract in Fao Cells Submitted to Oxidative Stress
- Protective effects of grape seed procyanidin extractagainst nickel sulfate-induced apoptosis and oxidative stress in rat testes
- Protective effects of oligomeric and polymeric procyanidin fractions from defatted grape seeds on tert-butyl hydroperoxide-induced oxidative damage in HepG2 cells
- Cocoa procyanidin chain length does not determine ability to protect LDL from oxidation when monomer units are controlled
- Critical Role of FoxO1 in Granulosa Cell Apoptosis Caused by Oxidative Stress and Protective Effects of Grape Seed Procyanidin B2
- The protective effect of grape seed procyanidin extract against cadmium-induced renal oxidative damage in mice
- Grape seed procyanidin extract attenuates hypoxic pulmonary hypertension by inhibiting oxidative stress and pulmonary arterial smooth muscle cells proliferation
- The Antioxidant Procyanidin Reduces Reactive Oxygen Species Signaling in Macrophages and Ameliorates Experimental Colitis in Mice
- Procyanidin B2 3,3″‐di‐O‐gallate induces oxidative stress‐mediated cell death in prostate cancer cells via inhibiting MAP kinase phosphatase activity and activating ERK1/2 and AMPK
- Procyanidins from Vitis vinifera Seeds: In Vivo Effects on Oxidative Stress
- Procyanidin dimer B1 and trimer C1 impair inflammatory response signalling in human monocytes
- Anti-inflammatory effects of grape seed procyanidinB2 on a diabetic pancreas
- Synthesis of Procyanidin B3 and Its Anti-inflammatoryActivity. The Effect of 4-Alkoxy Group of Catechin Electrophile in the Yb(OTf)3-Catalyzed Condensation with Catechin Nucleophile
- Anti-inflammatory effectof procyanidin B1 on LPS-treated THP1 cells via interaction with the TLR4–MD-2 heterodimer and p38 MAPK and NF-κB signaling
- Effect of Procyanidins on DOMS and Blood Plasma IL-6 in College Athletes
- Modulation of TNF-α Secretion in Peripheral Blood Mononuclear Cells by Cocoa Flavanols and Procyanidins
- Effects of Hawthorn Leaf Procyanidins on Over Expressions of ICAM-1 and E-selectin in Human Umbilical Vein Endothelial Cells Induced by TNF-α
psoralidin
- Diverse role of fast growing rhizobia in growth promotionand enhancement of psoralen content in Psoralea corylifolia L
- Phytopharmacological assessment of medicinal properties of Psoralea corylifolia
- ANTIMICROBIAL ACTIVITY OF LEAF EXTRACT OF PSORALEA CORYLIFOLIA L.
- Polyphenols Displaying Tyrosinase Inhibition from the Seed of Psoralea corylifolia
- Mechanism of Cytotoxicity by Psoralea corylifolia Extract in Human Breast Carcinoma Cells
- In vitro enhancement of psoralen as an important anticancer compound in Psoralea corylifolia through precursor feeding
- Psoralea corylifolia l. an endangered medicinal plant with broad spectrum properties
- Preliminary Phytochemical Studies and Evaluation of Antibacterial Activity of Psoralea corylifoliaSeed Extract
- Phytochemical Screening and Antibacterial Potentiality of Essential Oil from Psoralea corylifoliaLinn.
- ANTIMICROBIAL AND ANTIOXIDANT SYNERGY OF PSORALEA CORYLIFOLIALINN.ANDPLUMBAGOZEYLANICALINN.
- Dietary Total Prenylflavonoids from the Fruits of Psoralea corylifolia L. Prevents Age-Related Cognitive Deficits and Down-Regulates Alzheimer’s Markers in SAMP8 Mice
- Antimicrobial activity in fruit extract of Psoralea corylifoliaL. on pathogenic organisms
- Phenolic Compounds Isolated from Psoralea corylifolia Inhibit IL-6-induced STAT3 Activation
- Bavachin from Psoralea corylifolia Improves Insulin-Dependent Glucose Uptake through Insulin Signaling and AMPK Activation in 3T3-L1 Adipocytes
- The ethanol extraction of prepared Psoralea corylifolia induces apoptosisand autophagy and alteres genes expression assayed by cDNA microarray in human prostate cancer PC‐3 cells
- Compounds isolated from Psoralea corylifolia seeds inhibit protein kinase activity and induce apoptotic cell death in mammalian cells
- Characterization of Compounds in Psoralea corylifoliaUsing High-Performance Liquid Chromatography Diode Array Detection, Time-of-Flight Mass Spectrometry and Quadrupole Ion Trap Mass Spectrometry
- Psoralea corylifolia L. Attenuates Nonalcoholic Steatohepatitis in Juvenile Mouse
- Prenylated flavonoid‐standardized extract from seeds of Psoralea corylifolia L. activated fat browning in high‐fatdiet–induced obese mice
- Psoralea corylifolia extracts stimulate cholinergic-like psoralen receptors of tadpole-tail melanophores, leading to skin darkening
- Fabrication of anti-vitiligo ointment containing Psoralea corylifolia: in vitro and in vivo characterization
- Protective effects of the compounds isolated from the seed of Psoralea corylifolia on oxidative stress-induced retinal damage
- Psoralea corylifolia L. Seed Extract Ameliorates Streptozotocin-Induced Diabetes in Miceby Inhibition of Oxidative Stress
- Anti-oxidant modulation in response to gamma radiation induced oxidative stress in developing seedlings of Psoralea corylifolia L.
- Protective Role of Psoralea corylifolia L.Seed Extract against Hepatic Mitochondrial Dysfunction Induced by Oxidative Stress or Aging
- In Vivo Anti-diabetic and Anti-oxidant Potential of Psoralea corylifolia Seeds in Streptozotocin Induced Type-2 Diabetic Rats
- Neuroprotective effects of Psoralea corylifolia Linn seed extracts on mitochondrial dysfunction induced by 3-nitropropionic acid
- Prenylflavones from Psoralea corylifolia Inhibit Nitric Oxide Synthase Expression through the Inhibition of I-κB-α Degradation in Activated Microglial Cells
- Protective Effect of Psoralea corylifolia L. Seed Extract against Palmitate-Induced Neuronal Apoptosis in PC12 Cells
- Quantitative Analysis of Psoralea corylifolia Linne and its Neuroprotective and Anti-Neuroinflammatory Effectsin HT22 Hippocampal Cells and BV-2 Microglia

- Pterostilbene surpassed resveratrol for anti-inflammatoryapplication: Potency consideration and pharmacokinetics perspective
- Anti-inflammatory Action of Pterostilbene Is Mediated through the p38 Mitogen-Activated Protein Kinase Pathway in Colon Cancer Cells
- Effects of polyphenols including curcuminoids, resveratrol, quercetin, pterostilbene, and hydroxypterostilbene on lymphocyte pro-inflammatory cytokine production of senior horses in vitro
- Pterostilbene exerts an anti-inflammatory effect via regulating endoplasmic reticulum stress in endothelial cells
- Effect of resveratrol and pterostilbene on aging and longevity
- Pterostilbeneattenuates theinflammatory reaction induced by ischemia/reperfusion in rat heart
- The Inhibitory Effect of Pterostilbene on Inflammatory Responsesduring the Interaction of 3T3-L1 Adipocytes and RAW 264.7 Macrophages
- Pterostilbene inhibits inflammation and ROS production in chondrocytes by activating Nrf2 pathway
- Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity
- Pterostilbene and allopurinol reduce fructose-induced podocyte oxidative stress and inflammation via microRNA-377
- SIRT1 activation by pterostilbene attenuates the skeletal muscle oxidative stress injury and mitochondrial dysfunction induced by ischemia reperfusion injury
- Blueberry Component Pterostilbene Protects Corneal Epithelial Cells from Inflammation via Anti-oxidative Pathway
- Protective effect of Pterostilbene against free radical mediated oxidative damage
- Pterostilbene protects against myocardial ischemia/reperfusion injury via suppressing oxidative/nitrative stress and inflammatory response
- Pterostilbene reduces oxidative stress, prevents hypertrophy and preserves systolic function of right ventricle in cor pulmonale model
- Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis
- Pterostilbene ameliorates insulin sensitivity, glycemic control and oxidative stress in fructose-fed diabetic rats
- Pterostilbene attenuates high glucose-induced oxidative injuryin hippocampal neuronal cells by activating nuclear factor erythroid 2-related factor 2
- Evidence for oxidative detoxication of pterostilbene and resveratrol by a laccase-like stilbene oxidase produced by Botrytis cinerea
- Pterostilbene, a natural dimethylated analog of resveratrol, inhibits rat aortic vascular smooth muscle cell proliferation by blocking Akt-dependent pathway
- Pterostilbene suppresses vascular adhesion molecule expression in TNF-α-stimulated vascular muscle cells
- Resveratrol and pterostilbene inhibit TNF-α stimulated activation of NF-kB and its upstream TAK1 in macrophages via modulation of sphingolipids
- Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-κB pathway in lipopolysaccharide-stimulated macrophage
- Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages
- The Flavonoid Quercetin Inhibits Proinflammatory Cytokine (Tumor Necrosis Factor Alpha) Gene Expression in Normal Peripheral Blood Mononuclear Cells via Modulation of the NF-κβ System
- Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line
- Quercetin Reduces Inflammatory Pain: Inhibition of Oxidative Stress and Cytokine Production
- Quercetin Ingestion Does Not Alter Cytokine Changesin Athletes Competing in the Western States Endurance Run
- A comparison of the effects of kaempferol and quercetinon cytokine-induced pro-inflammatory status of cultured human endothelial cells
- The flavonoid, quercetin, differentially regulates Th-1 (IFNγ) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells
- Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-κB pathway
- Quercetin Is More Effective than Cromolyn in Blocking Human Mast Cell Cytokine Release and Inhibits Contact Dermatitis and Photosensitivity in Humans
- Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect
- Quercetin protects mouse liver against triptolide-induced hepatic injury by restoring Th17/Treg balance through Tim-3 and TLR4-MyD88-NF-κB pathway
- Quercetin Inhibits Inflammatory Bone Resorption in a Mouse Periodontitis Model
- Tannic acid and quercetin display a therapeutic effect on atopic dermatitisvia suppression of angiogenesis and Th2 polarization. (97.7)
- Protective role of quercetin against cisplatin-induced hair cell damage in zebrafish embryos
- INHIBITION BY QUERCETIN OF THE PROMOTING EFFECT OF TELEOCIDIN ON SKIN PAPILLOMA FORMATION IN MICE INITIATED WITH 7, 12-DIMETHYLBENZ[a]ANTHRACENE
- Quercetin protects against hair cell loss in the zebrafish lateral line and guinea pig cochlea
- Quercetin, Inflammation and Immunity
- The Inhibitory Effect of Quercetin on IL-6 Production by LPS- Stimulated Neutrophils
- Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammationin mice via the NF-κB pathway
- Quercetin abrogates IL-6/STAT3signaling and inhibits glioblastoma cell line growth and migration
- Quercetin inhibits IL-1 beta-induced ICAM-1 expression in pulmonary epithelial cell line A549 through the MAPK pathways
- Quercetin regulates Th1/Th2 balance in a murine model of asthma
- In vivo quercitrin anti‐inflammatory effect involves release of quercetin, which inhibits inflammation through down‐regulation of the NF‐κB pathway
- Quercetin Inhibits IL-1β-Induced Proliferation and Production of MMPs, COX-2, and PGE2 by Rheumatoid Synovial Fibroblast
- Anti-inflammatory activityof quercetin and isoquercitrin in experimental murine allergic asthma
- Quercetin 3-O-β-(2″-galloyl)-glucopyranoside inhibits endotoxin LPS-induced IL-6 expression and NF-κB activation in macrophages
- Regulation of IL‐1‐induced selective IL‐6 release from human mast cells and inhibition by quercetin
- Quercetin Down-regulates IL-6/STAT-3 Signals to Induce Mitochondrial-mediated Apoptosis in a Nonsmall- cell Lung-cancer Cell Line, A549
- Regulatory mechanisms of IL-2 and IFNγ suppression by quercetin in T helper cells
- Quercetin, a bioflavonoid, accelerates TNF-α-induced growth inhibition and apoptosis in MC3T3-E1 osteoblastic cells
- Quercetin accelerates TNF-α-induced apoptosis of MC3T3-E1 osteoblastic cells through caspase-dependent and JNK-mediated pathways
- Effect of apoE genotype and dietary quercetin on blood lipids and TNF-α levels in apoE3 and apoE4 targeted gene replacement mice
- Quercetin-3-O-(2″-galloyl)-α-l-rhamnopyranoside inhibits TNF-α-activated NF-κB-induced inflammatory mediator production by suppressing ERK activation
- Human Quercetin Conjugated Metabolites Attenuate TNF-α-induced Changes in Vasomodulatory Molecules in an HUASMCs/HUVECs Co-culture Model
- Effects of Quercetin in IL-6 and TNF-α Levels in Diabetic Rats
- Anti-inflammatory property of quercetin through downregulation of ICAM-1 and MMP-9 in TNF-α-activated retinal pigment epithelial cells
- The inhibitory effects of the flavonoid quercetin on endotoxin (LPS)-stimulated production of tumor necrosis factor-α (TNF-α) by normal human peripheral blood mononuclear cells (PBMC)
- Regulation of IL‐1‐induced selective IL‐6 release from human mast cells and inhibition by quercetin
- Quercetin inhibits the poly(dA:dT)-induced secretion of IL-18 via down-regulation of the expressions of AIM2 and pro-caspase-1 by inhibiting the JAK2/STAT1 pathway in IFN-γ-primed human keratinocytes
- Quercetin inhibits IL-1β induced ICAM-1 expression in human alveolar epithelial cells.
- PENGARUH QUERCETIN TERHADAP KADAR INTERLEUKIN-1 (IL-1) TIKUS YANG DIINDUKSI OBAT ANTI TUBERKULOSI
- Quercetin inhibits epithelial–mesenchymal transition, decreases invasiveness and metastasis, and reverses IL-6 induced epithelial–mesenchymal transition, expression of MMP by inhibiting STAT3 signaling in pancreatic cancer cells
- Inhibitory effects of quercetin on LPS–stimulated IL–6 production by neutrophils
- Effects of Quercetin on Muscle Function during IL-6-induced Cancer Cachexia
- EFFECT OF QUERCETIN ON STRESS INDUCED CHANGES IN INTERLEUKIN 6 (IL-6) LEVELS IN ALBINO RATS.
- QUERCETIN INHIBITS IL-6/JAK/STAT3 SIGNALLING PATHWAY IN LIVER OF RATS WITH CIRRHOSIS INDUCED BY COMMON BILE DUCT LIGATION
- Inhibitory mechanism of quercetin 3-0-beta -(2 “-galloyI )-glucopyranoside on IL-6 expression in LPS -stimulated macrophages RAW 264 .7
- Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia
- Quercetin suppresses inflammation by reducing ERK1/2 phosphorylation and NF kappa B activation in Leptin-induced Human Umbilical Vein Endothelial Cells (HUVECs)
- Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway
- Quercetin Suppresses the Migration and Invasion in Human Colon Cancer Caco-2 Cells Through Regulating Toll-like Receptor 4/Nuclear Factor-kappa B Pathway
- Quercetin Protects Mice from ConA-Induced Hepatitis by Inhibiting HMGB1-TLR Expression and Down-Regulating the Nuclear Factor Kappa B Pathway
- Hepatoprotective Effect of Quercetin on Endoplasmic Reticulum Stress and Inflammation after Intense Exercise in Mice through Phosphoinositide 3-Kinase and Nuclear Factor-Kappa B
- Quercetin ameliorates peripheral nerve ischemia–reperfusion injury through the NF-kappa B pathway
- Induction of apoptosis and NF-$\kappa$B by quercetin in growing murine L1210 lymphocytic leukaemic cells potentiated by TNF
- Quercetin Mitigates Inflammatory Responses Induced by Vascular Endothelial Growth Factor in Mouse Retinal Photoreceptor Cells through Suppression of Nuclear Factor Kappa B
- Quercetin and Vitamin C Mitigate Cobalt Chloride-Induced Hypertension through Reduction in Oxidative Stress and Nuclear Factor Kappa Beta (NF-Kb) Expression in Experimental Rat Model
- Impact of Quercetin, Diallyl Disulfide and Nimbolide on the Regulation of Nuclear Factor Kappa B Expression in Prostate and Breast Cancer Cell Lines
- Quercetin Ameliorates NO Production via Down-regulation of iNOS Expression, NFκB
Activation and Oxidative Stress in LPS-Stimulated Macrophages - EFFECT OF QUERCETIN AND GLIBENCLAMIDE COMBINATION ON CARDIAC MUSCLE NUCLEAR FACTOR KAPPA B (NF-KB) EXPRESSION IN TYPE 2 DIABETIC RATS
- Effects of Quercetin and Omega-3 Combination on Nuclear Factor Kappa B (NFκB) Expression Level in Pancreatic Tissue of Rats with Type-2 Diabetes Mellitus

Radix Angelicae
- Data Analysis for Different Proportions in Clinic Application of Radix Angelicae sinensis and Radix Astragali
- An herbal decoction of Radix astragali and Radix angelicae sinensis promotes hematopoiesis and thrombopoiesis
- A Chinese Herbal Decoction, Danggui Buxue Tang, Prepared from Radix Astragali and Radix Angelicae Sinensis Stimulates the Immune Responses
- Hematopoietic effect of Radix angelicae sinensis in a hemodialysis patient
- Chemical and Biological Assessment of a Traditional Chinese Herbal Decoction Prepared from Radix Astragali and Radix Angelicae Sinensis: Orthogonal Array Design to Optimize the Extraction of Chemical Constituents
- Identification of Antioxidants in Essential Oilof Radix Angelicae Sinensis Using HPLC Coupled with DAD-MS and ABTS-Based Assay
- Protective effect of Danggui (Radix Angelicae Sinensis) on angiotensin II-induced apoptosis in H9c2 cardiomyoblast cells
- Differentiation of human adipose-derived stem cells into neuron-like cells by Radix Angelicae Sinensis
- Radix Angelicae Sinensis and Radix Hedysari enhance radiosensitivity of 12C6+ radiation in human liver cancercells by modulating apoptosis protein
- Anti-inflammatory effect of radix Angelicae sinensis
- Z-ligustilide isolated from Radix Angelicae sinensis ameliorates the memory impairment induced by scopolamine in mice
- Two glutathione S‐transferase inhibitors from Radix Angelicae sinensis
- Anti-inflammatory Effect of Radix Angelicae Sinensis
- Effect of Propranolol on Attenuation of Rat Hypoxic Pulmonary Hypertension due to Radix Angelicae Sinensis
- Component Analysis of SFE-CO_2 Extracts of Radix Angelicae sinensis,Rhizoma Chuanxiong and Their Drug Partnership by GC-MS
- Correlation between Antioxidant Activities and Phenolic Contents of Radix Angelicae Sinensis (Danggui)
- Chemical and Biological Assessment of a Chinese Herbal Decoction Containing Radix Astragali and Radix Angelicae Sinensis: Determination of Drug Ratio in Having Optimized Properties
- Perimenopause Amelioration of a TCM Recipe Composed of Radix Astragali, Radix Angelicae Sinensis, and Folium Epimedii: An In Vivo Study on Natural Aging Rat Model
- Effects of radix Angelicae sinensis on hemorrheology in patients with acute ischemic stroke.
- [Effects of Radix angelicae sinensis on systemic and portal hemodynamics in cirrhotics with portal hypertension].
- [Effect of radix Angelicae sinensis on serum gastrin levels in patients with cirrhosis].
- Effects of Radix Angelicae Sinensis, Radix Astragali and their compatiblities on Platelet aggregation and changes of cAMP and cGMP in platelet
- A Chinese Herbal Decoction, Dang Gui Bu Xue Tang, Prepared from Radix Astragali and Radix Angelicae sinensis, Ameliorates Insulin Resistance Induced by A High-Fructose Diet in Rats
- A Chinese Herbal Decoction Prepared from Radix Astragali and Radix Angelicae Sinensis Induces the Expression of Erythropoietinin Cultured Hep3B Cells
- Effects of Radix Angelicae sinensisand shuanghuanglian on a rat model of chronic Pseudomonas aeruginosa pneumonia.
- Effects of Radix Angelicae Sinensis and Rhizoma Chuanxiong on Mouse Uterine Contractions in Vitro
- [Adjustment effect of Radix Astragalus and Radix Angelicae sinensis on TNF-alpha and bFGF on renal injury induced by ischemia reperfusion in rabbit].
- Effect of Volatile Oil of Radix Angelicae Sinensison Percutaneous Absorption of Ferulic Acid
- [Study on the dynamic variations of Z-ligustilide and n-butylidenephthalide content in essential oil of radix angelicae sinensis from different growth period].
- Effect of Ultrasonic Extraction on Chemical Compositions in Fluid Extract of Radix Angelicae Sinensis
- Effects of Radix Angelicae Sinensis on changes of IL-6, TNF-? and bFGF in renal ischemia/reperfusion injury in rabbits

Rehmannia glutinosa
- Rehmannia glutinosa Activates Intracellular Antioxidant Enzyme Systems in Mouse Auditory Cells
- Protective effect of Rehmannia glutinosa on the cisplatin-induced damage of HEI-OC1 auditory cells through scavenging free radicals
- [Cloning and expression analysis of a tyrosine decarboxylase gene from Rehmannia glutinosa].
- Rehmannia glutinosa: Tissue Culture and Its Potential for Improvement
- Simultaneous Analysis of Bioactive Metabolites from Rehmannia glutinosa by HPLC-DAD-MS/MS
- QUALITY ASSURANCE OF CHINESEHERBAL DRUGS CONSIDERING ASEXAMPLE REHMANNIA GLUTINOSAAND STEPHANIA TETRANDRA
- Sorting and identification of Rehmannia glutinosagermplasm resources based on EST-SSR, scanning electron microscopy micromorphology, and quantitative taxonomy
- Rehmannia glutinosa exhibits anti‐aging effect through maintaining the quiescence and decreasing the senescence of hematopoietic stem cells
- Composition containing composite extract of rehmannia glutinosa and pueraria lobata for preventing or treating menopausal symptoms
- The enhanced immune response of PCV-2 vaccine using Rehmannia glutinosa polysaccharide liposome as an adjuvant
- Rehmannia glutinosa polysaccharide promoted activation of human dendritic cells
- Rehmannia Glutinosa Pharmacopuncture Solution Regulates Functional Activation, FcεRI Expression, and Signaling Events in Mast Cells
- Puffing of Rehmannia glutinosa enhances anti-oxidantcapacity and down-regulates IL-6 production in RAW 264.7 cells
- Rehmannia glutinosa polysaccharide functions as a mucosal adjuvant to induce dendritic cell activation in mediastinal lymph node
- Anti-inflammatory Activities of Ethylacetate Extract of Rehmannia glutinosa in LPS-induced RAW 264.7 Cells
- Effect of Rehmannia glutinosaon immediate type allergic reaction
- Rehmannia glutinosa Suppresses Inflammatory Responses Elicited by Advanced Glycation End Products
- Rehmannia glutinosa (Gaertn.) DC. polysaccharide ameliorates hyperglycemia, hyperlipemia and vascular inflammation in streptozotocin-induced diabetic mice
- Immunoenhancement effect of rehmannia glutinosa polysaccharide on lymphocyte proliferation and dendritic cell
- Hot Water Extracted Lycium Barbarum and Rehmannia Glutinosa Inhibit Liver Inflammationand Fibrosis in Rats
- Bioassay-guided isolation of anti-inflammatory components from the root of Rehmannia glutinosa and its underlying mechanism via inhibition of iNOS pathway
- Rehmannia glutinosa ameliorates scopolamine-induced learning and memory impairment in rats.
- Topical application of Rehmannia glutinosa extract inhibits mite allergen-induced atopic dermatitisin NC/Nga mice
- Plasma Glucose Lowering Mechanisms of Catalpol, an Active Principle from Roots of Rehmannia glutinosa, in Streptozotocin-Induced Diabetic Rats
- Rehmannia glutinosa ameliorates the progressive renal failure induced by 5/6 nephrectomy
- Characterization of the Anti-Diabetic and Antioxidant Effects of Rehmannia Glutinosa in Streptozotocin-Induced Diabetic Wistar Rats
- Antidiabetic and antioxidant effects of catalpol extracted from Rehmannia glutinosa (Di Huang) on rat diabetesinduced by streptozotocin and high-fat, high-sugar feed
- The Effects of Rehmannia glutinosa on the Protein Expression Related to the Angiogenesis, Cell Survival and Inflammation
- Treatment of Primary Chronic Glomerulonephritis with Rehmannia Glutinosa Acteosides in Combination with the Angiotensin Receptor Blocker Irbesartan: A Randomized Controlled Trial
- Protective effect of Rehmannia glutinosa on the cisplatin-induced damage of HEI-OC1 auditory cells through scavenging free radicals

- Anti-inflammatory Effect of Resveratrol and Polydatin by In Vitro IL-17 Modulation
- Decreased severity of experimental autoimmune encephalomyelitis during resveratroladministration is associated with increased IL-17+IL-10+ T cells, CD4− IFN-γ+ cells, and decreased macrophage IL-6 expression
- Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts
- Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function
- Anti-inflammatory effect of resveratrol in old mice liver
- Resveratrol ameliorates diabetes-induced renal damage through regulating the expression of TGF-β1, collagen IV and Th17/Treg-related cytokines in rats
- Anti-Inflammatory Effects of Resveratrol, Curcumin and Simvastatin in Acute Small Intestinal Inflammation
- Resveratrol-Mediated Gamma Interferon Reduction Prevents Airway Inflammation and Airway Hyperresponsiveness in Respiratory Syncytial Virus-Infected Immunocompromised Mice
- Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice
- Regulatory role of resveratrol on Th17 in autoimmune disease
- ResveratrolAmeliorates Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice
- Alteration of Hepatic Proinflammatory Cytokines is Involved in the Resveratrol-Mediated Chemoprevention of Chemically-Induced Hepatocarcinogenesis
- Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice
- Resveratrolprotects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2–Keap1 signaling
- Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic β‐cell dysfunction in streptozotocin‐nicotinamide‐induced diabetic rats
- Resveratrol retards progression of diabetic nephropathy through modulations of oxidative stress, proinflammatory cytokines, and AMP-activated protein kinase
- One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease
- Inhibition of nitric oxide and inflammatory cytokines in LPS-stimulated murine macrophages by resveratrol, a potent proteasome inhibitor
- Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure in streptozotocin–nicotinamide-induced experimental diabetic rats
- Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats
- Resveratrol Attenuates CoCl2-Induced Cochlear Hair Cell Damage through Upregulation of Sirtuin1 and NF-κB Deacetylation
- Inhibitory Effects of Resveratrol on Melanin Synthesis in Ultraviolet B-Induced Pigmentation in Guinea Pig Skin
- Resveratrol Ameliorates Dysregulation of Th1, Th2, Th17, and T Regulatory Cell-Related Transcription Factor Signaling in a BTBR T + tf/J Mouse Model of Autism
- Resveratrol prevents suppression of regulatory T-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet–induced obesity
- Anti-inflammatory Effects of Resveratrol and Its Potential Use in Therapy of Immune-mediated Diseases
- Amelioration of oxidative stress by antioxidants and resveratrol in PC12 cells
- Resveratrol increases vascular oxidative stressresistance
- Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats
- Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells
- Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells
- Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: Effects on the inhibition of STAT3 phosphorylation
- Resveratrol induces apoptosis, influences IL-6 and exerts immunomodulatory effect on mouse lymphocytic leukemia both in vitro and in vivo
- Resveratrol Inhibits Respiratory Syncytial Virus-Induced IL-6 Production, Decreases Viral Replication, and Downregulates TRIF Expression in Airway Epithelial Cells
- Resveratrol inhibits IL‐6‐induced ovarian cancer cell migration through epigenetic up‐regulation of autophagy
- Resveratrol, a sirtuin 1 activator, increases IL-6 production by peripheral blood mononuclear cells of patients with knee osteoarthritis
- Resveratrol Inhibits IL-6-Induced Transcriptional Activity of AR and STAT3 in Human Prostate Cancer LNCaP-FGC Cells
- Trans-Resveratrol Attenuates High Fatty Acid-Induced P2X7 Receptor Expression and IL-6 Release in PC12 Cells: Possible Role of P38 MAPK Pathway
- Resveratrol reduces IL-6 and VEGF secretion from co-cultured A549 lung cancer cells and adipose-derived mesenchymal stem cells
- Inhibitory effects of resveratrol on MCP-1, IL-6, and IL-8 production in human coronary artery smooth muscle cells
- Resveratrol inhibits the IL-1β-induced expression of MMP-13 and IL-6 in human articular chondrocytes via TLR4/MyD88-dependent and -independent signaling cascades
- Function of sustained released resveratrol on IL-1β-induced hBMSC MMP13 secretion inhibition and chondrogenic differentiation promotion
- Resveratrol inhibition of TNF-α and IL-1 for treatment of rheumatoid arthritis: from In-Silico to In-vitro elucidation
- Resveratrol Protects against Sepsis-Associated Encephalopathyand Inhibits the NLRP3/IL-1?Axis in Microglia
- RESVERATROL, THE ANTIOXIDANT OF RED WINE,PREVENTS IL-1 MEDIATED MITOCHONDRIALDYSFUNCTION AND ATP DEPLETION: ROLE OF PGE2AS A CAUSE OF CELLULAR ENERGY DEPLETION
- Effect of resveratrol on the expression of TNF-α and IL-1 in dorsal root ganglion neuron after auto-transplantation of nucleus pulposus in rat model
- PENGARUH RESVERATROL TERHADAP KADAR IL-5, EOSINOFIL DARAH, %VEP 1 DAN SKOR ACT PENDERITA ASMA
- Resveratrol inhibits nitric oxide and TNF-α production by lipopolysaccharide-activated microglia
- Resveratrol attenuates TNF-α-induced activation of coronary arterial endothelial cells: role of NF-κB inhibition
- Anti-inflammatory effect of resveratrol on TNF-α-induced MCP-1 expression in adipocytes
- Resveratrol inhibits TNF-α-induced changes of adipokines in 3T3-L1 adipocytes
- Resveratrol Inhibits TNF-α–Induced Proliferation and Matrix Metalloproteinase Expression in Human Vascular Smooth Muscle Cells
- Resveratrol prevents TNF-α-induced muscle atrophy via regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes
- Resveratrol inhibits TNF-α-induced IL-1β, MMP-3 production in human rheumatoid arthritis fibroblast-like synoviocytes via modulation of PI3kinase/Akt pathway
- RV09, a novel resveratrol analogue, inhibits NO and TNF-α production by LPS-activated microglia
- Resveratrol is an effective inducer of CArG-driven TNF-α gene therapy
- Resveratrol enhances TNF-α production in human monocytes upon bacterial stimulation
- The anti-inflammatory activity of the polyphenol resveratrol may be partially related to inhibition of tumour necrosis factor-α (TNF-α) pre-mRNA splicing
- Inhibition of TNF‐α‐mediated endothelial cell–monocyte cell adhesion and adhesion molecules expression by the resveratrol derivative, trans‐3,5,4′‐trimethoxystilbene
- Resveratrol Protects against TNF-α-Induced Injury in Human Umbilical Endothelial Cells through Promoting Sirtuin-1-Induced Repression of NF-KB and p38 MAPK
- Resveratrol Reduces TNF-α-induced U373MG Human Glioma Cell Invasion through Regulating NF-κB Activation and uPA/uPAR Expression
- Effect of cis-resveratrol on genes involved in nuclear factor kappa B signaling
- Resveratrol inhibits VEGF expression of human hepatocellular carcinoma cells through a NF-kappa B-mediated mechanism.
- Effect of resveratrol on activation of nuclear factor kappa-B and inflammatory factors in rat model of acute pancreatitis
- Resveratrol Enhances Radiosensitivity of Human Non-Small Cell Lung Cancer NCI-H838 Cells Accompanied by Inhibition of Nuclear Factor-Kappa B Activation
- Resveratrol Inhibits Nitric Oxide-Induced Apoptosis via the NF-Kappa B Pathway in Rabbit Articular Chondrocytes
- Protective effects of chronic resveratrol treatment on vascular inflammatory injury in streptozotocin-induced type 2 diabetic rats: Role of NF-kappa B signaling
- Resveratrol Attenuates Oxidative Stress Induced by Balloon Injury in the Rat Carotid Artery Through Actions on the ERK1/2 and NF-Kappa B Pathway
- The effects of resveratrol on cyclooxygenase-1 and -2, nuclear factor kappa beta, matrix metalloproteinase-9, and sirtuin 1 mRNA expression in hearts of streptozotocin-induced diabetic rats
- Resveratrol mitigates hepatic injury in rats by regulating oxidative stress, nuclear factor-kappa B, and apoptosis
- Genetically Engineered Resveratrol-Enriched Rice Inhibits Neuroinflammation in Lipopolysaccharide-Activated BV2 Microglia Via Downregulating Mitogen-Activated Protein Kinase-Nuclear Factor Kappa B Signaling Pathway
- Effects of resveratrol on NF Kappa B modulation and squamous cell proliferation
- Abstract 1025: Resveratrol Attenuates Oxidative/Nitrative Stress in Diabetes Mellitus by inhibiting TNF alpha induced NF kappa B activation
- Inhibition of the Expression of Matrix Metalloproteinases in Articular Chondrocytes by Resveratrol through Affecting Nuclear Factor-Kappa B Signaling Pathway

Sanguisorba officinalis
- Sanguisorba Officinalis Root Extract Has FGF-5 Inhibitory Activity and Reduces Hair Loss by Causing Prolongation of the Anagen Period
- Use of at least one extract of a rosacea of the genus Sanguisorba officinalis for promoting pigmentation of the skin and/or the body hair and/or the cranial hair
- The Inhibitory Effect of an Extract of Sanguisorba officinalis L. on Ultraviolet B-Induced Pigmentationvia the Suppression of Endothelin-Converting Enzyme-1α
- Synergistic topical application of salt-processed Phellodendron amurense and Sanguisorba officinalis Linnealleviates atopic dermatitis symptoms by reducing levels of immunoglobulin E and pro-inflammatory cytokines in NC/Nga mice
- Burn wound healing potential of a polysaccharide from Sanguisorba officinalis L. in mice
- Promotion of anagen, increased hair density and reduction of hair fall in a clinical setting following identification of FGF5-inhibiting compounds via a novel 2-stage process
- Healing effect of Sanguisorba officinalis Lextract on second-degree burns in rats
- Sanguisorba officinalis extract, ziyuglycoside I, and II exhibit antiviral effects against hepatitis B virus
- Inhibitory Effect of Sanguisorba officinalis Extract on Lipopolysaccharide-induced Pro-inflammatory Events in Macrophages
- In vivo antiviral activitiy of Sanguisorba officinalis rootsagainst viral hemorrhagic septicemia virus in olive flounder Paralichthys olivaceus
- Chemical constituents from Sanguisorba officinalis L. and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells
- ANTI-ALLERGIC EFFECTS OF SANGUISORBA OFFICINALIS ON ANIMAL MODELS OF ALLERGIC REACTIONS
- Anti-allergic inflammatory components from Sanguisorba officinalis L
- Ethanol extracts of Sanguisorba officinalis L. suppress TNF-α/IFN-γ-induced pro-inflammatory chemokineproduction in HaCaT cells
- A hot‐water extract of Sanguisorba officinalis ameliorates endotoxin‐induced septic shock by inhibiting inflammasome activation
- Inhibitory effect of Sanguisorba officinalis ethanol extract on NO and PGE2 production is mediated by suppression of NF-κB and AP-1 activation signaling cascade
- Anti-asthmatic effect of Sanguisorba officinalis L.and potential role of heme oxygenase-1 in an ovalbumin-induced murine asthma model
- Anti-Wrinkle Activity of Ziyuglycoside I Isolated from a Sanguisorba officinalis Root Extract and Its Application as a Cosmeceutical Ingredient
- Methanol extract of Sanguisorba officinalis L.with cytotoxic activity against PC3 human prostate cancer cells
- The Inhibitory Effect of Triterpenoid GlycosidesOriginating from Sanguisorba officinalis on Tissue Factor Activity and the Production of TNF-α
- Chemical Composition, Antioxidant and Antimicrobial Activities of Sanguisorba officinalis L. Extracts
- Triterpenoid saponins of Sanguisorba officinalisand their anti-inflammatory activity
- Sanguisorba officinalis L. ExtractsExert Antiobesity Effects in 3T3-L1 Adipocytes and C57BL/6J Mice Fed High-Fat Diets
- Ethanol extract of Sanguisorba officinalis L. inhibits biofilm formation of methicillin-resistant Staphylococcus aureus in an ica-dependent manner
- Apoptotic effectof hot water extract of Sanguisorba officinalis L. in human oral cancer cells
- Determination of free and bounded phenolic acids in the rhizomes and herb of Sanguisorba officinalis L.
- Extracts of the medicinal herb Sanguisorba officinalisinhibit the entry of human immunodeficiency virus-1
- Saponins from Sanguisorba officinalis Improve Hematopoiesis by Promoting Survival through FAK and Erk1/2 Activation and Modulating Cytokine Production in Bone Marrow
- Sanguisorba officinalis L synergistically enhanced 5-fluorouracil cytotoxicity in colorectal cancer cells by promoting a reactive oxygen species-mediated, mitochondria-caspase-dependent apoptotic pathway
- Antioxidant Activity and Antimicrobial Effect for Foodborne Pathogens from Extract and Fractions of Sanguisorba officinalis L.
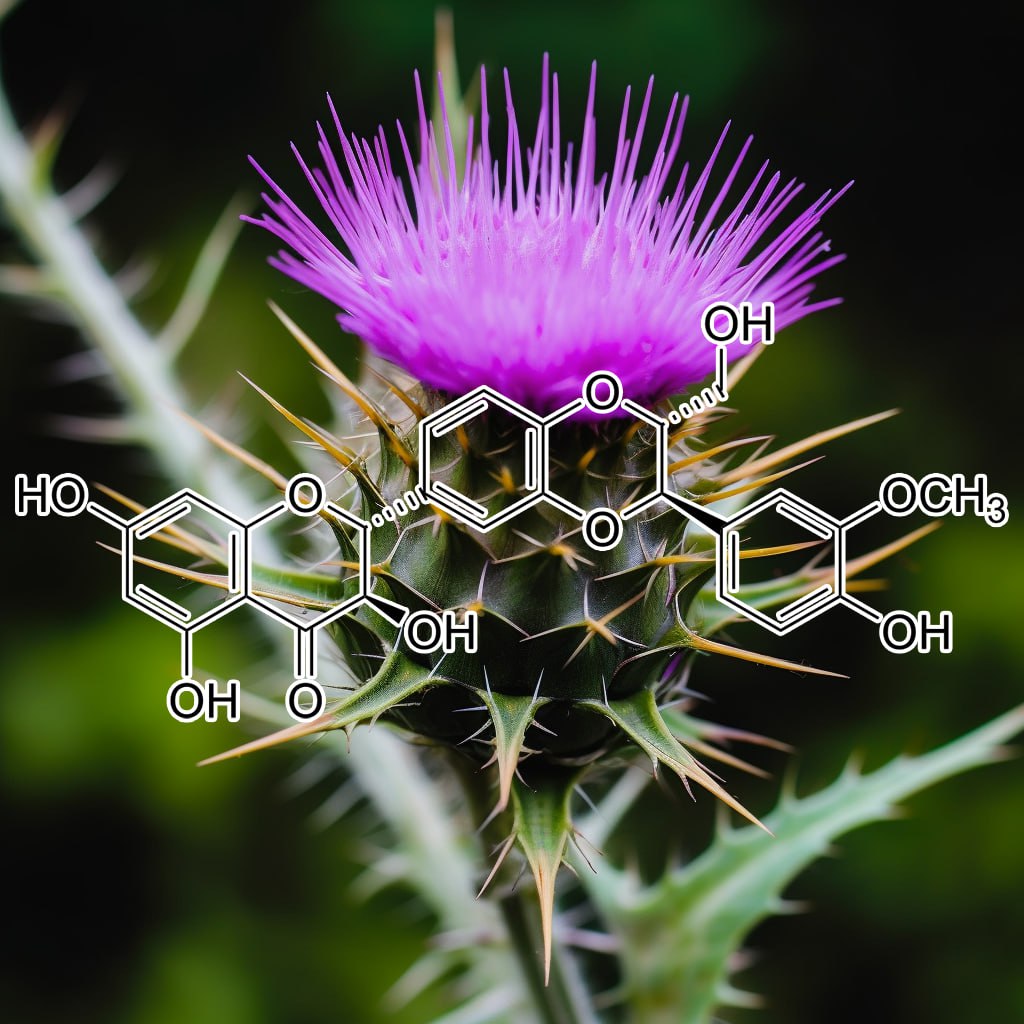
- Silibinininhibits cytokine-induced signaling cascades and down-regulates inducible nitric oxide synthase in human lung carcinoma A549 cells
- Growth Inhibition and Regression of Lung Tumors by Silibinin: Modulation of Angiogenesis by Macrophage-Associated Cytokines and Nuclear Factor-κB and Signal Transducers and Activators of Transcription 3
- Silibinin modulates the NF-κb pathway and pro-inflammatory cytokine production by mononuclear cells from preeclamptic women
- Hesperidin and Silibinin Ameliorate Aluminum-Induced Neurotoxicity: Modulation of Antioxidants and Inflammatory Cytokines Level in Mice Hippocampus
- Silibinin inhibits the production of pro-inflammatory cytokines through inhibition of NF-κB signaling pathway in HMC-1 human mast cells
- Silibinin attenuates oxidative metabolism and cytokine production by monocytes from preeclamptic women
- Silibinin attenuates allergic airway inflammation in mice
- Silibinin Inhibits Inflammatory and Angiogenic Attributes in Photocarcinogenesis in SKH-1 Hairless Mice
- Silibinin alleviates inflammationand induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes and has a therapeutic effect on arthritis in rats
- Effects of Silibinin on Hair Follicle Stem CellsDifferentiation to Neural-like Cells
- Oxaliplatin-Induced Neuropathy: Oxidative Stress as Pathological Mechanism. Protective Effect of Silibinin
- Hydrogel containing silibinin-loaded pomegranate oil based nanocapsules exhibits anti-inflammatory effects on skin damage UVBradiation-induced in mice
- Silibinin Inhibits Tumor Promotional Triggers and Tumorigenesis Against Chemically Induced Two-Stage Skin Carcinogenesis in Swiss Albino Mice: Possible Role of Oxidative Stress and Inflammation
- Role of p53 in silibinin-mediated inhibition of ultraviolet B radiation-induced DNA damage, inflammation and skin carcinogenesis
- Neurotrophic Effects of Silibinin on Differentiation of Hair Follicle Stem Cells to Neurons
- Neurotrophic Effects of Silibinin on Differentiation of Hair Follicle Stem Cells to Neurons
- Flavonoid Silibinin Increases Hair-Inductive PropertyVia Akt and Wnt/β-Catenin Signaling Activation in 3-Dimensional-Spheroid Cultured Human Dermal Papilla Cells
- Silibinin inhibits ultraviolet B radiation‐induced mast cells recruitment and bone morphogenetic protein 2 expression in the skin at early stages in Ptch(+/−) mouse model of basal cell carcinoma
- Behavioral and neurochemical deficits in aging rats with increased neonatal iron intake: silibinin’s neuroprotection by maintaining redox balance
- Silibinin prevents amyloidbpeptide-inducedmemory impairment and oxidative stress in mice
- Silibinin Prevents Autophagic Cell Death upon Oxidative Stress in Cortical Neurons and Cerebral Ischemia-Reperfusion Injury
- Silibinin Attenuates Amyloid β25–35 Peptide-Induced Memory Impairments: Implication of Inducible Nitric-Oxide Synthase and Tumor Necrosis Factor-α in Mice
- Silibinin inhibits ultraviolet B radiation‐induced DNA‐damage and apoptosis by enhancing interleukin‐12 expression in JB6 cells and SKH‐1 hairless mouse skin
- Silibinin strongly inhibits the growth kinetics of colon cancer stem cell-enriched spheroids by modulating interleukin 4/6-mediatedsurvival signals
- Silymarin inhibits UV radiation-induced immunosuppression through augmentation of interleukin-12 in mice
- Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats
- Silibinin Attenuates Sulfur Mustard Analog-Induced Skin Injury by Targeting Multiple Pathways Connecting Oxidative Stress and Inflammation
- Silibinin potentially attenuates arsenic-induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats
- Silibinin Is a Potent Sensitizer of UVA Radiation‐induced Oxidative Stress and Apoptosis in Human Keratinocyte HaCaT Cells
- Silibinin ameliorates oxidative stress induced aberrant crypt foci and lipid peroxidation in 1, 2 dimethylhydrazine inducedrat colon cancer
- Silibinin Inhibits the Invasion of IL-6-Stimulated Colon Cancer Cells via Selective JNK/AP-1/MMP-2 Modulation in Vitro
- Chemopreventive Effects of Silibinin on Colitis-Associated Tumorigenesis by Inhibiting IL-6/STAT3 Signaling Pathway
- Silibinin protects OTA‐mediated TNF‐α release from perfused rat livers and isolated rat Kupffer cells
- Silibinin Suppresses TNF-α-Induced MMP-9 Expression in Gastric Cancer Cells through Inhibition of the MAPK Pathway
- Silibinin modulates TNF‐α and IFN‐γ mediated signaling to regulate COX2 and iNOS expression in tumorigenic mouse lung epithelial LM2 cells
- Apoptosis Induction by OTA and TNF-α in Cultured Primary Rat Hepatocytes and Prevention by Silibinin
- Silibinin down-regulates FAT10 and modulate TNF-α/IFN-γ-induced chromosomal instability and apoptosis sensitivity
- Silibinin Improves TNF-α and M30 Expression and Histological Parameters in Rat Kidneys After Hepatic Ischemia/Reperfusion
- Silibinin modulates lipid homeostasis and inhibits nuclear factor kappa B activation in experimental nonalcoholic steatohepatitis
- Downregulation of nuclear factor-kappa B (NF-κB) pathway by silibinin in human monocytes challenged with Paracoccidioides brasiliensis
- Silibinin inhibits invasive properties of human glioblastoma U87MG cells through suppression of cathepsin B and nuclear factor kappa B-mediated induction of matrix metalloproteinase 9
- Silibinin Induces Apoptosis and Inhibits Proliferation of Estrogen Receptor (ER)-Negative Breast Carcinoma Cells through Suppression of Nuclear Factor Kappa B Activation

Sophora flavescens
- The hair growth promoting effect of Sophora flavescensextract and its molecular regulation
- Rhizobial Diversity and Nodulation Characteristics of the Extremely Promiscuous Legume Sophora flavescens
- Improvement of androgenetic alopecia with topical Sophora flavescens Aiton extract,and identification of the two active compounds in the extract that stimulate proliferation of human hair keratinocytes
- Studies on the effect of Sophora flavescens extract on the hair growth stimulation and acne inhibition
- Ultrasound-assisted extraction of oxymatrine from Sophora flavescens
- Study on the Induction and Culture Condition of Hairy Roots of Sophora flavescens Ait
- Effect of Sophora flavescens alkaloid on aerobic vaginitis in gel form for local treatment
- A New Lavandulylated Flavonoid from Sophora flavescensRoots
- Induction of Hairy Rootsand Determination of Oxymatrine in Sophora flavescens Ait.
- Preventive and Therapeutic Effects of Traditional Medicines on Diabetic Complications: Sophora flavescens,Pueraria lobata, Coptis chinensis, Cirsium maackii, and Artemisia capillaries
- Anti-inflammatory Activity of Trifolirhizin isolated from Methanol Extractof Sophora Flavescens Ait (the Sophorae Radix) in Experimental Animals
- Prenylated Flavonoids from the Roots of Sophora flavescens Promote Vascular Relaxation via Endothelium-Dependent Mechansims
- Sophora flavescens Seed as a promising high potential by-product: Phytochemical characterization and bioactivity evaluation
- Sophoraflavanone G from Sophora flavescens induces apoptosis in triple-negative breast cancer cells
- Efficiency of Sophora flavescens-Fructus Ligustri Lucidi Drug Pairs in the Treatment of Liver Fibrosis Based on the Response Surface Method
- Anti-inflammatory and anti-arthritic activity of total flavonoids of the roots of Sophora flavescens
- Anti-Inflammatory and Antiproliferative Activities of Trifolirhizin, a Flavonoid from Sophora flavescens Roots
- Attenuation of ERK/RSK2-Driven NFκB Gene Expression and Cancer Cell Proliferation by Kurarinone, a Lavandulyl Flavanone Isolated from Sophora flavescens Ait. Roots
- Sophora flavescens Aitoninhibits the production of pro-inflammatory cytokines through inhibition of the NF κB/IκB signal pathway in human mast cell line (HMC-1)
- Anti-inflammatory effect of sophoraflavanoneG isolated from Sophora flavescens in lipopolysaccharide-stimulated mouse macrophages
- Anti-inflammatory and Anti-oxidant Effects of Sophora flavescens Root Extraction in Lipopolysaccharide-activated Raw 264.7 Cells.
- Hepatoprotective and inhibiting HBV effects of polysaccharides from roots of Sophora flavescens
- In vivo and in vitro anti-inflammatory effects of Sophora flavescens residues
- Simultaneous quantification of multiple alkaloids in Sophora Flavescens Ait and human urine by HPLC
- The Effect of Allergic Inflamation by Sophora FlavescensAiton Extract Ion Through Inhibition of the NFκB, JNK and p38 Pathway
- Sophora flavescens protects against mycobacterialTrehalose Dimycolate-induced lung granuloma by inhibiting inflammation and infiltration of macrophages
- Antibacterial and Anti-inflammatory Activityof Traditional Chinese Herb Pairs, Angelica sinensis and Sophora flavescens
- Anti-inflammatory effects of fermented and non-fermented Sophora flavescens: a comparative study
- Effect of Sophora flavescens Aiton extract on degranulation of mast cells and contact dermatitis induced by dinitrofluorobenzene in mice
- Anti‐Inflammatory and PPAR Transactivational Properties of Flavonoids from the Roots of Sophora flavescens
- The Effect of Allergic Inflamation by Sophora Flavescens Aiton Extract Ion Through Inhibition of the NFκB
, JNK and p38 Pathway - Sophora Flavescens Suppresses Degranulation and Pro-inflammatory Cytokines Production through the Inhibition of NF-κB
(p65) Activation in the RBL-2H3 cells

Eriodictyon angustifolium
Reduction in human hair graying by sterubin, an active flavonoid of Eriodictyon angustifolium
Hair pigmentation could be improved by altering melanocyte number and gene activity. Cytokines such as Kitl and Endothelins are known to promote the proliferation of melanocytes and upregulate their pigmentation genes. Recently, the WNT/β catenin produced by the follicular keratinocytes was found to function as an attractant of follicular melanocytes and activate microphthalmia-associated transcription factor (MITF), the master regulator of pigmentation genes of melanocytes. Based on these notions, many synthetic or natural compounds, including plant extracts, that can upregulate pigmentation genes have been screened. Depletion of follicular MSCs causes persistent loss of functional melanocytes from the hair matrix leading to graying, with no recovery expected. Gray hair-containing follicles that are depleted of MSCs increase along with the cumulative progression of the hair cycle with age.
Loss of proliferative ability of follicular keratinocytes, rather than MSCs, has also been reported in radiation-induced hair graying suggesting the involvement of keratinocytes in hair graying and their potential as a practical target for its prevention. Thus, owing to the complexity of the mechanisms underlying hair graying, a combination of in vitro and in vivoevaluation methods is necessary for screening effective molecules for the prevention and reduction of hair graying.
Sterubin is an active anti-inflammatory component of Yerba Santa (Eriodictyon angustifolium) Yerba Santa is a plant that grows on the west coast of North America and has been used for several years as a traditional medicine by the indigenous population. To investigate its ability to stimulate melanogenesis, we treated the human melanoma cell line HMVII with 5–10 μM of synthetic sterubin. The sterubin-treated cells showed 1.47- to 1.65-fold concentration-dependent increase (**p < 0.01) in melanin content after 7 days of exposure (Fig. 1A). Immunohistochemistry revealed nuclear translocation of β-catenin, which is indicative of WNT signaling activation, and nearly 1.4-fold increase in nuclear MITF expression (**p < 0.01) and 4.6-fold increase in cytoplasmic TYR expression (***p < 0.001) were observed in the treated cells (Fig. 1B-E).

Age-associated hair graying and radiation-induced hair graying have been suggested to share common cellular mechanisms [
9]. In addition, follicular keratinocytes that provide a niche for melanocytes have been suggested to have indispensable roles for age-associated and radiation-induced hair graying [
Next, using the 2′,7′-dichlorofluorescein diacetate (DCF-DA) assay, we investigated whether sterubin could scavenge radiation-induced intracellular ROS. After exposure to 20 Gy of X-ray, increased fluorescence intensity of DCF was observed in NHEKs (DMSO-treated cells in Fig. 2A). As shown in Fig. 2A and B, 100 μM of synthetic sterubin significantly suppressed the radiation-induced generation of ROS from 33.4 to nearly the level observed in non-irradiated control cells (Sterubin + 20 Gy: 13.7, ***p < 0.001). ROS attack mitochondrial DNA and membrane proteins, thereby impairing mitochondrial function. Mito-tracker staining revealed that 100 μM of synthetic sterubin treatment for 1 h before irradiation significantly prevented severe deformation of the mitochondrial mass of NHEKs (DMSO + 20 Gy: 46.3, Sterubin + 20 Gy : 71.8, **p < 0.01) (Fig. 2C, D). These data suggest that sterubin contains active components that could effectively prevent hair graying by protecting follicular keratinocytes from age- and radiation-induced damage.

Thuja orientalis
- Hair growth-promoting activity of hot water extract of Thuja orientalis
- Free radical scavenging and antielastase activities of flavonoids from the fruits of Thuja orientalis
- Isoquercitrin is the most effective antioxidant in the plant Thuja orientalis and able to counteract oxidative-induced damage to a transformed cell line (RGC-5 cells)
- Chemical Composition and Fungitoxic Activity of Essential Oil of Thuja orientalis L. Grown in the North-Western Himalaya
- Biosorption of Reactive Blue 49 dye under batch and continuous mode using a mixed biosorbent of macro-fungus Agaricus bisporus and Thuja orientalis cones
- Biflavonyl pigments from [the foliage of] Thuja orientalis
- Molluscicidal activity of Saraca asoca and Thuja orientalis against the fresh water snail Lymnaea acuminata
- Inhibitory effect of Thuja orientalis on TNF‐α‐induced vascular inflammation
- The mating system in natural populations of Thujaorientalis
- Protective effects of standardized Thuja orientalis leaves against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells
- GC analysis of essential oils in the rumen fluid after incubation of Thuja orientalis twigs in the Rusitec system
- A new labdane diterpenoid with anti-inflammatory activity from Thuja orientalis
- Antiviral Activity of the Plant Extracts from Thuja orientalis, Asterspathulifolius, and Pinus thunbergii Against Influenza Virus A/PR/8/34
- Response of vegetative growth and chemical constituents of Thuja orientalis L. plant to foliar application of different amino acids at Nubaria.
- Improved biosorption potential of Thuja orientalis cone powder for the biosorptive removal of Basic Blue 9
- Effects of Flooding of Soll on Growth, Stem Anatomy, and Ethylene Production of Thuja Orientalis Seedlings·
- Antibacterial, antioxidant andphytochemical investigation of Thuja orientalisleaves
- Anti-allergic effect of lambertianic acid from Thuja orientalis in mouse bone marrow-derived mast cells
- Larvicidal Activity of Chamaecyparis obtusa and Thuja orientalis Leaf Oils against Two Mosquito Species
- Flavonoids from the leaves of Thuja orientalis inhibit the aldose reductase and the formation of advanced glycation endproducts
- ANTIOXIDANT POTENTIAL OF VARIOUS EXTRACTS OF STEM OF THUJA ORIENTALIS: IN VITRO STUDY
- Three New Diterpenoids from the Leaves of Thuja orientalis
- ANTIMICROBIAL ACTIVITY OF THE ESSENTIAL OIL OF THUJA ORIENTALIS L
- Methylene chloride fraction of the leaves of Thuja orientalis inhibits in vitro inflammatory biomarkers by blocking NF-κB and p38 MAPK signaling and protects mice from lethal endotoxemia
- Efficiency of Thuja orientalis and Artimisia campestris extracts to control of Potato leaf roll virus (PLRV) in potato plants.
- Effect of the Semen Extract of Thuja orientalis on Inflammatory Responses in Transient Focal Cerebral Ischemia Rat Model and LPS-Stimulated BV-2 Microglia
- Batch and Dynamic Flow Biosorption Potential of Agaricus bisporus/Thuja orientalis Biomass Mixture for Decolorization of RR45 Dye
- Histopathology of Thuja orientalis and Juniperus horizontalis plumosa Infected with Meloidogyne incognita
- Chemical composition of Thuja orientalis L. essential oils and study of their allelopathic potential on germination and seedling growth of weeds
- Thuja orientalis reduces airway inflammation in ovalbumin-induced allergic asthma
- Inhibitory activity of Thuja orientalis on TNF-α-induced vascular cell adhesion in HUVECs

- Celastrol inhibits pro-inflammatory cytokine secretion in Crohn’s disease biopsies
- Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-κB in LPS-stimulated BV-2 microglial cells
- CELASTROL, A POTENT ANTIOXIDANT AND ANTI-INFLAMMATORY DRUG, AS A POSSIBLE TREATMENT FOR ALZHEIMER’S DISEASE
- Celastrol ameliorates murine colitis via modulating oxidative stress, inflammatory cytokinesand intestinal homeostasis
- Celastrol ameliorates cytokine toxicity and pro-inflammatory immune responses by suppressing NF-κB activation in RINm5F beta cells
- Suppression ofinflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii
- Modulation ofinflammatory signaling and cytokine release from microglia by celastrol incorporated into dendrimer nanocarriers
- Celastrol suppresses allergen-induced airway inflammation in a mouse allergic asthma model
- Celastrus-derived Celastrol Suppresses Autoimmune Arthritis by Modulating Antigen-induced Cellular and Humoral Effector Responses
- Suppression of autoimmune arthritis by Celastrus-derived Celastrol through modulation of pro-inflammatory chemokines
- Modulatory effect of celastrolon Th1/Th2 cytokines profile, TLR2 and CD3+ T-lymphocyte expression in a relapsing–remitting model of multiple sclerosis in rats
- CelastrolAttenuates Hypertension-Induced Inflammation and Oxidative Stressin Vascular Smooth Muscle Cells via Induction of Heme Oxygenase-1
- Celastrol nanomicelles attenuate cytokine secretion in macrophages and inhibit macrophage-induced corneal neovascularization in rats
- Celastrol Alleviates Airway Hyperresponsiveness and Inhibits Th17 Responses in Obese Asthmatic Mice
- Control of autoimmune inflammation by celastrol, a natural triterpenoid
- Celastrol mediates Th17 and Treg cell generation via metabolic signaling
- Celastrol alleviates arthritis by modulating the inflammatory activities of neutrophils
- Celastrol Limits Autoimmune Arthritis by Reducing Th17 and Promoting Treg Cells in Inflamed Joints
- CelastrolAlleviates Airway Hyperresponsiveness by Downregulating Th17in Obese Asthmatic Mice
- Celastrol attenuates oxidative stress in the skeletal muscle of diabetic rats by regulating the AMPK-PGC1α-SIRT3 signaling pathway
- Celastrol reduces IL-1β induced matrix catabolism, oxidative stress and inflammation in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration in vivo
- Celastrol Blocks Interleukin-6 Gene Expression via Downregulation of NF-κB in Prostate Carcinoma Cells
- Celastrol inhibits interleukin-17A-stimulated rheumatoid fibroblast-like synoviocyte migration and invasion through suppression of NF-κB-mediated matrix metalloproteinase-9 expression
- Therapeutic potential of interleukin-17 in inflammation and autoimmune diseases
- Celastrolameliorates experimental colitis in IL-10 deficient mice via the up-regulation of autophagy
- Celastrol inhibits IL-1β-induced inflammation in orbital fibroblasts through the suppression of NF-κB activity
- Celastrol treatment protects against acute ischemic stroke-induced brain injury by promoting an IL-33/ST2 axis-mediated microglia/macrophage M2 polarization
- Celastrol Prevents Atherosclerosis via Inhibiting LOX-1 and Oxidative Stress
- Celastrol protects mouse retinas from bright light-induced degeneration through inhibition of oxidative stress and inflammation
- Celastrol attenuates mitochondrial dysfunction and inflammation in palmitate-mediated insulin resistance in C3A hepatocytes
- Clinical evaluation of tripterygium wilfordii polyglycosidium combined with methotrexate in treating rheumatoid arthritis and its effect on TNF-α and IL-6.
- Inhibition of IL-1 release from human monocytes and suppression of streptococcal cell wall and adjuvant-induced arthritis in rats by an extract of Tripterygium wilfordii Hook
- Inhibitory effects of monomer T4 from Tripterygium wilfordii hook on cultured mesangial cells proliferation and IL-1 production
- Effects of Tripterygium wilfordii on the Production and Activities of IL- 1 and IL- 2 in vitro
- Effect of Tripterygium Wilfordii Hook f. on the Serum IL-6 and TNF Levels in Patients with Chronic Nephritis
- The effects of cyclosporin A(CyA) and Tripterygium wilfordii on the expressions of IL 6 and IL 8 mRNA of human peripheral blood mononuclear cells(PBMCs) induced by recombinant human IL 1α(rhIL 1α)
- Effect of Tripterygium wilfordii combined with methotrexate on short-term effect of rheumatoid arthritis and the levels of IL-6, IL-10 and TNF- in peripheral blood
- EFFECTS OF CELASTROL ON IL-1 AND lL-2 PRODUCTION
- Celastrol Inhibits Lung Infiltration in Differential Syndrome Animal Models by Reducing TNF-α and ICAM-1 Levels while Preserving Differentiation in ATRA-Induced Acute Promyelocytic Leukemia Cells
- The effect of Celastrol on the expressions of RANKL,OPG,IL-6,TNF-α and IL-8 in human rheumatoid synoviocyte MH7A
- The NF-kappa B inhibitor, celastrol, could enhance the anti-cancer effect of gambogic acid on oral squamous cell carcinoma
- NF-Kappa B Modulation Is Involved in Celastrol Induced Human Multiple Myeloma Cell Apoptosis
- Celastrol Inhibits Lipopolysaccharide-Induced Angiogenesis by Suppressing TLR4-Triggered Nuclear Factor-Kappa B Activation
- Synergism between NF-kappa B inhibitor, celastrol, and XIAP inhibitor, embelin, in an acute myeloid leukemia cell line, HL-60
- Triptolide Inhibits the Differentiation of Th17 cells and Suppresses Collagen‐induced Arthritis
- Th17/Treg imbalance in triptolide-induced liver injury
- [Triptolide inhibites Th17 cell differentiation via regulating cyclooxygenase-2/ prostaglandin E2 axis in synovial fibroblasts from rheumatoid arthritis].
- Triptolide ameliorates IL-10-deficientmice colitis by mechanisms involving suppression of IL-6/STAT3 signaling pathway and down-regulation of IL-17
- Quercetin protects mouse liver against triptolide-induced hepatic injury by restoring Th17/Treg balancethrough Tim-3 and TLR4-MyD88-NF-κB pathway
- Interleukin-17 mediates triptolide-induced liver injury in mice
- Triptolide protects mice from ischemia/reperfusion injury by inhibition of IL-17 production
- Triptolide ameliorates ileocolonic anastomosis inflammation in IL-10 deficient mice by mechanism involving suppression of miR-155/SHIP-1 signaling pathway
- Epigallocatechin-3-gallate Prevents Triptolide-Induced Hepatic Injury by Restoring the Th17/Treg Balance in Mice
- [Modulatory effect of triptolide on differentiation of human Th17 cells].
- Triptolide attenuates idiopathic pneumonia syndrome in a mouse bone marrow transplantation model by down-regulation of IL-17
- Triptolide suppresses proinflammatory cytokine-induced matrix metalloproteinase and aggrecanase-1 gene expression in chondrocytes
- Triptolide Inhibits Proliferation and Migration of Colon Cancer Cells by Inhibition of Cell Cycle Regulators and Cytokine Receptors
- Triptolide Attenuates Endotoxin- and Staphylococcal Exotoxin-Induced T-Cell Proliferation and Production of Cytokines and Chemokines
- Inhibition by Triptolide of Chemokine, Proinflammatory Cytokine, and Adhesion Molecule Expression Induced by Lipopolysaccharide in Corneal Fibroblasts
- Triptolide attenuates oxidative stress, NF-κB activation and multiple cytokine gene expression in murine peritoneal macrophage
- Inhibitory Effect of Triptolide on Chemokine Expression Induced by Proinflammatory Cytokines in Human Corneal Fibroblasts
- Triptolide induces anti-inflammatorycellular responses
- Triptolide, A Novel Immunosuppressive and Anti-Inflammatory Agent Purified from a Chinese Herb Tripterygium Wilfordii Hook F
- Preparation and anti-inflammatory activity of triptolide ethosomes in an erythema model
- Therapeutic effects of triptolide via the inhibition of IL-1β expression in a mouse model of ulcerative colitis
- Acute and subacute toxicity studies on triptolide and triptolide-loaded polymeric micelles following intravenous administration in rodents
- Interleukin 6 inhibition by triptolide prevents inflammation in a mouse model of ulcerative colitis
- Immunosuppressant PG490 (Triptolide) Inhibits T-cell Interleukin-2 Expression at the Level of Purine-box/Nuclear Factor of Activated T-cells and NF-κB Transcriptional Activation
- Triptolide suppresses T-lymphocyte proliferation by inhibiting interleukin-2 receptor expression, but spares interleukin-2 productionand mRNA expression
- The suppressive effect of triptolide on chronic colitis and TNF-α/TNFR2 signal pathway in interleukin-10deficient mice
- Inhibitory effect of triptolide on interleukin-18and its receptor in rheumatoid arthritis synovial fibroblasts
- Therapeutic effects of triptolide on interleukin-10 gene-deficient mice with colitis
- Triptolide suppresses interleukin-1β-inducedhuman β-defensin-2 mRNA expression through inhibition of transcriptional activation of NF-κB in A549 cells
- Interleukin-6-independent expression of glucocorticoid receptor is upregulated by triptolide in multiple myeloma
- Triptolide inhibits TNF-α, IL-1β and NO production in primary microglial cultures
- Triptolide inhibits amyloid-β1-42-induced TNF-α and IL-1β production in cultured rat microglia
- Triptolide inhibits COX-2 expression by regulating mRNA stability in TNF-α-treated A549 cells
- Effect of triptolide on secretion of inflammatory cellular factors TNF-α and IL-8 in peritoneal macrophages of mice activated by lipopolysaccharide
- Relationship between single nucleotide polymorphism in TNF-α gene promoter region and inibitory effects of triptolide on TNF-α production in peripheral blood mononuclear cells of healthy humans
- Triptolide inhibits the function of TNF‑α in osteoblast differentiation by inhibiting the NF‑κB signaling pathway
- The Studies on Triptolide and TNF-α Regulating the Expression of Notch1 in Raji Cells

Ursolic acid

Urtica Dioica
Mandatory FDA Disclaimer:
As with any supplement, consult your medical practitioner prior to using this product if you are pregnant, may become pregnant, breastfeeding, taking medication, other dietary supplements or have a medical condition.
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. *Daily Value has not been established.
| Size | 100g |
|---|







Shelly –
Wow! I am truly amazed at how quickly my scalp stopped itching only a few days of using this product! Gavin and his crew are stellar in what they do, I am a lifetime customer. This new Super HairTonic will not leave you disappointed! Once again, Interstellar crushed it!!!! Thank you for always providing quality top notch products!
Chad –
I ordered Super tonic hair as soon as it became available. First off, the taste when mixed into coffee makes the coffee taste better. This product is one you will look forward to buying again and again. After using for a week not only did my energy levels increase, but also my hair seems to be shiny, thicker and generally easier to work with. I wish that I had this product a year ago. Would’ve saved me a lot of money buying other hair products that didn’t work well. What an extraordinary product! Thank you Interstellar!! For the quality products and customer service!!!!
Rich Ryan –
I’ve always had thin hair and after about age 40 I started getting more and more hair in my combs and brushes. I wasn’t going bald, but my hair was breaking very easily. Haircuts became optional as my hair would only grow to shoulder length before breaking off. My hair and beard felt brittle and dry, and the skin underneath my beard dried out and itched constantly unless treated with something. Also most of my beard is grey now. I can see SuperHair slowly improving all these things. There’s less hair in my comb, my hair and beard feels softer and less brittle, and my scalp and facial skin under the beard feels healthier and itches considerably less. The grey hasn’t reversed yet, but I’ve only been on the blend for a couple weeks. I’ll post an update in a year or so when the blend has had a chance to work. Once again, an incredible product from Interstellar Blends!
Tanya –
I’ve been waiting a little to post a review on this product as I try to provide as much honest and authentic detail as possible. I have never been that girl with amazing hair. I’m not saying it’s bad but it’s fine and lacks volume. Combine that with thyroid issues and it had been dry and broken in the past. I’ve been on this blend about a month and this past week I’ve started to have great hair days. (Previously I may have had one great hair day per year! My hair is fuller, has more volume and shine. I’m totally sold on this blend. This one does take a little time I think – so give it an honest go
Soma Felfoldi (verified owner) –
First of all, I would just like to say that Im extremely grateful to Gavin! I have been in this health field for almost half a decade and tried numerous supplements on the market but I barely got any reaction to them sadly. I already started experimenting with the dry fast thank to this site and since my Peel, Super hair and pine pollen arrived the whole dry fasting became just so easy that I feel like I never want to go back to eating. And its only 9-10 days that I have been using them! What will happen after spending some months on the protocol? Holy Moly Im excited!! After I take the blends my brain just clears out, and I am able to use the brain just like when I was in my high school years- sharp, quick math calculations, more talkative and more convincing which is very important at my work! Since mentally Im working better it seems I can do much better at my hobby which is DIY little works for homes and carpentering. I totally believe that hands skills are coordinated by the brain and my improved performance is thanks to the mental clarity.
The main reason why I got into the blends and protocol is that I have hair and skin issues since the age of 17. Now Im 27 and I dont want to shout too early but the nasty dermatitis seems to fade away finally from my face. I cant pinpoint exactly which herb does help with that but I dont even have to. I trust Gavin and the combo blends are created to work synergestically so they cannot not work! I always knew fruits and fruits skins are healthy but were never able to eat enough of them due to getting bloating. Now finally I can take the active substance from the fruits without getting bloated and this removed a huge mental burden from me that I finally get my anti oxidants. Since stressing used to increase my dermatitis and I havent had a flare up since the last 10 days!
I still have way to go on this health journey as my body temperature is lower than it should be and Im very interested in the Thermo blend and 1-2 others suggested by some members of the dry fasting group. I know my protocol is not complete yet but if its working already this well, I cant wait what will happen once I could get my hands on some more blends! Gavin, thank you for setting up such a supportive community which gives people hope and finally something which is rarely achieved: success and results!
Paula DeNote Zering –
I am a hairstylist. I have my own salon and have been doing hair for over 25 years. My hair is semi fine, breaks easy and of course I want it longer. I have been on the blends for other reason as well. I take several but added Super Tonic Hair for the sole purpose of it not falling out when I lose weight. Today my daughter was applying my color and asked me what was going on with the front of my hair. It is usually all white. I told her I was taking the blends and it’s supposed to help with grey. She laughed and said I now have dark hairs growing where there was all white a few months ago. She couldn’t believe it. Neither could I. While this is great for some, we need to keep this on the down low! Lol! I make my living on coloring white hair back. Just kidding. This stuff is amazing!
Amelia Jara –
I strongly believe that God is uncovering things like Interstellar blends to prepare our minds for the tough time we are facing. People are full of anxiety and fear that release toxins in the brain and damage it and consequently not able to think straight and make right choices. I have been practicing fasting for many years for the positive effect it does to my mind, and for weight lost . However, it was very hard to do and the pounds I lost would come back right after I resume my eating.. I started using interstellar blend in July 2019 and it’s been one of the most amazing thing I have done in my life. They are autophagy activating herbs. Auto means self and phagy means eating. Those herbs activate a system in the body that takes broken proteins ,cells that are weak and bacteria’s that are creating problems and metabolize them and feeds the body with them.. Even though you are not eating, your body is feeding you breakfast , lunch and dinner with the raw material it contains . I love all the blends but the ones that I am the most excited about are Autophagy, Super Hair, pine pollen and Niagara. Autophagy suppresses my hunger like crazy for the same reason I just stated. My body is feeding me. Isn’t that awesome. I am always calm and my stress level is very low .. Niagara’s is awesome. My libido was very low because of problems with my hormones. The first week after I started using Niagara , my intimacy with my husband increased tremendously ,sex libido and stamina increased and painful sex disappeared. Super hair combined with pine pollen are amazing. I have very dry and curly hair that never grow. Now my hair is growing and is more manageable . I also see some black hair coming out of my white hair. When I first called Gavin, he immediately sent me some samples of Thermo, nebula,trinity, peel, spice , Seven Sages ,Shilajit and matcha green tea. The first week using them revolutionized my entire body and soul. I was losing so much weight that my husband started to complain. I moved from size medium to small and extra small. My pants size are from youth 3 to 5 and that’s because I eat a lot when I eat because my husband doesn’t like it when I am too skinny. Almost a month after I started using the herbs I went to a resort at the beach eating 3 huge meals a day and snacking and drinking in between meals for 10 days and when I came back to my normal fasting routine with the blends , the first week my body looked like if I didn’t go on vacation. I am always with a strong sense of hope and good expectations for the future. I don’t get tired or sick. Praise God! I added ACB and Luteolin. My mind is so sharp that I can dream for a better future with the expectancy of living a long and happy life. Every week I either do 88/8 or 66/6 which means don’t eat food for 88 hours and just take the blends every 4 hours , then feed for 8 hours. Then the other 2 days I do intermitting fasting. This is life saving for me. I lose a lot of weight , my mind is sharp at work and I don’t have to go to the stress of preparing food to take to work. Now I am not worried
about gaining weight. My body only craves for healthy food and I am never tempted to eat the unhealthy food. My body is free from pain and inflammation and I am always looking forward to fast with the interstellar blends because of the sense of strength and encouragement I experience during the fast. I feel the presence of God in a strong way and I feel that I am stronger than the problems I face. I am always waiting for the next day with joy and positive expectations . Your body is your best friend that will be with you for the rest of your life and it is the vehicle that takes you to the places that bring you satisfaction and fulfillment in life. Therefore give it the best. Give it Intersteller blends. Your body will thank you and rejoice with you.
Rochelle Vitler (verified owner) –
I started using Auto, Thermo, Trinity, Super Hair and Pine Pollen approximately six weeks ago to support my overall health, mood and weight loss journey whilst undertaking a combination of 48-72 hour dry and water fasts.
I had already been fasting for approximately two months at this point but almost straightaway after introducing the blends the fasting became a breeze. I have the energy and inclination to exercise every day; I enjoy a big meal every two to three days and the desire to eat outside of these times is simply not there.
I have played around with the timing and combination of blends that works best for me and now believe I’ve got it just right. The best way to describe my mental state at the moment is “joyful”. I honestly feel happy, hopeful and uplifted, regardless of what stressors may be present in my life, and definitely attribute this to the blends.
Added bonus: I have new hair growth all around my hairline. I feel my hair overall is thicker and in much better condition but the hairline is particularly noticeable.
I can’t wait to try the next batch of blends I have ordered and will gradually add others. These blends really have made a big difference to my life and after seeing the benefits I wouldn’t want to be without them.
Claudia Gonzalez (verified owner) –
I purchased Superhair Tonic back in December. I had been water fasting (without blends) since August and managed to lose 30 lbs. However, by December I noticed I had started to shed tons of hair. Taking a shower caused severe anxiety since its when I saw the most hair fall out. Hand fulls of my long 30 inch hair were falling out. I had just come across the Facebook group and saw this blend. I ordered immediately. I took this combined with the Pine Pollen and within two weeks all hair loss had stopped. I was so amazed and thrilled.
I think the water fasting has also stunned my hair growth. I hadn’t seen much growth in the last couple of months. After a couple of weeks on the Superhair Tonic and Pine Pollen I noticed that my hair was growing again. I documented the growth and from the end of December to February my hair grew 3 inches! Not only that but I have tons of baby hair already growing in replacing all the hair I lost. I was afraid I would have to chop off my waist long hair with all the hair loss. But I am so happy to report that these wonderful blends have saved my hair and the 3 years that I have dedicated to growing and caring for it.
I also wanted to talk about Niagra. I’m 34 years old and have always had issues with irregular menstrual cycles. Early on they diagnosed me with PCOS. However, recently, with blood work they couldn’t really determine if that was the cause. I could go even 6-8 months without a period. I also started taking Niagra early in January. I was amazed that shortly after this I had a menstrual cycle, and then 34 days later another! No cramping, no pain, just a minor discomfort which I appreciated to know it was coming. I am so wowed at how fast this acted on regulating my cycle. I’ve felt great and will continue to take this.
I am taking other blends, like Trinity and Luteolin at nigh and my sleep has been wonderful. I truly recommend all the blends!
Qaz (verified owner) –
Ok finally I get to do a review! I discovered interstellar blends 3 months ago when I was searching about a certain herb. At first I thought this website was too good to be true but I looked at the hundreds of good reviews and also the reviews people have left on Facebook and I knew I had to give this a try. Now I am lucky to have great hair genetics in my family both sides of my family have a great head of hair and my dad who 52 still has a full head of hair. For me sadly I still have my full hair of hair but my scalp gets oily in one day which makes my hair brittle and fine. I’ve been taking the super hair tonic for only 3 weeks along with pine pollen and OMG, the greasy hair hasn’t gone completely yet but There’s a big improvement and it’s only been 3 weeks! Not to mention how much energy pine pollen gives me as its such a powerful super food. It’s best to take both of them together in warm water. Looking forward to trying your other blends Thank you so much a Gavin !!!
Nick K (verified owner) –
I’ve been taking the super hair blend as well as the spice & peel blends for about a month now.
Im impressed with the ingredient list, first and foremost. I’ve tried everything from pills to masks for my hair, and nothing comes close to this ingredient profile.
The flavor is amazing, similar to a herbal tea.
Mixing it with the Spice and Peel makes it that much better. I make it with hot water and drink it first thing in the morning.
Since taking it, I’ve noticed an increase in both thickness and shine. I’m in the process of growing my hair out, and this is one step I now realize I need to get my hair where I want it to be. I’m excited to see the progress continue over the next few months, pairing the blends with fasting and an antioxidant based diet.
anil badhan (verified owner) –
Amazing product, worth every penny! From the first tea I made, I felt amazing and gave me some goosebumps. The guys sent me sample of trinity also, which i combined both herbs together which made the experience way better and will be purchasing trinity next!
After few days using the herbs, I noticed how shiny my hair got. Every time I had a shower, my hair used to fall out, during and drying it. I’ve tried so many shampoos, conditioners, hair masks and oils to prevent the hair loss. This product is the best by far that I have used for hair loss.
I would recommend this to anyone with hair problems (one guy has already placed an order). Super happy how things are going and can’t wait to see the results at the end of the herbs. 100% returning back to buy more herbs! Thank you guys ! Cant wait to try new herbs! Life time customer !
Enough Love
Gloria Ho (verified owner) –
I love this blend. It is so easy to take this with any of my drinks twice daily. As of today, I have used the product for two weeks and have noticed a change in my hair and scalp. I do hard labor, so I sweat often and at the end of the night, I feel that my scalp is dry and itchy. When it does, hair falls out. I have tried numerous products and nothing works as well as this blend.
Since using the super hair formula, I’ve noticed my hair have stopped falling out. In addition, I’ve noticed that the necessity of my hair to be washed is less frequent because I do no experience scalp dryness or itchiness anymore. I know that washing my hair every day induces the falling out of hair, and since using this product, I can now wash my hair less frequently as well as seeing less hair fall out of my head. I really love that I can see the results right away and don’t have to take multiple pills a day in hopes that my hair would grow out. I’ve also noticed a bit of hair growth, and see baby hairs growing where there was an area in my hairline receding.
This formula is super easy to take. Every morning I mix it with my coffee and drink it easily and it does not leave a bad aftertaste. It really blends well with coffee. I also take it later in the day with any drink and I like that after today, I can reduce the intake amount. I’m very happy I got this blend and that it’s actually working. You come so many products that advertise online and promise you hair growth and you purchase it for ridiculous amount of money and subscribe to them, taking them over the.months without seeing results. With this super hair formula, I was able to feel and see the results almost immediately and am speechless with the end result.
I will definitely be a returning customer and continue to use this product alongside Pine Pollen which I also bought. This prevents hair loss and replaces so many products such as pills, shampoos, conditioners, and other home remedies I’ve tried. It is by far the best hair loss product I’ve ever used. Thank you to Interstellar for the research and effort that they have put into in order to create such a fantastic blend for hair. I can’t wait to try the other blends. I’ve also got the Triinity, Thermo, and Supernova blends, and they are fantastic as well and helps me with intermittent fasting! Thank you so much for creating this product and finding such a great blend of herbs! I’m excited to get my hair where I want it to be.
Best Regards! You’ll be hearing from me soon with additional purchases! Fantastic work you’ve done here!
Nirali (verified owner) –
First thing First, Interstellar blends are a game changer. I ordered Super Hair Tonic and have been taking it for about 3 weeks now and I can already see the difference.I have Wavy curls, and my hair were pretty well until I moved to Canada. After moving to Canada, I started experiencing grey hair, Hair-Fall, low hair volume and my hair started to break very easily. I tried so many supplements and hair care products but all in vain. I was so frustrated and stopped caring about my hair at all.
Then my friend introduced me to Interstellar Blends. I reached out to Gavin and he recommended Super Tonic Hair. I was very skeptical at first as everything i’ve tried until now had shown no result but I thought of giving it a try. The order came in just few days of ordering it. I have been taking the Super Tonic Hair for 3 weeks and I can literally see the difference in my hair. They are now getting longer, less hair-fall and I can also see new hairs growing. And its not just about the hair but after I started taking Super Tonic Hair, my whole body feels so different, Very light and always at ease. This blends are really life changing and I can’t thank Gavin enough for creating this blend. He is very knwoledgable and answers any question that I have right away. The Customer service is very SPOT-ON.
Over-all I am very happy with the result and I am really glad that I gave interstellar blends a try. I can’t wait to explore other blends and reset my body.
I have just become the lifetime customer of Interstellar Blends.
jennifer (verified owner) –
I have always had good hair, thick and wavy. but it doesn’t seem to grow much past shoulders. And despite having a good diet and pampering my hair with ‘good ” hair products, it would still get a bit dry and frizzy… after a month of using the Super Hair Tonic, my hair grew, I’m seeing new hair growth and its not dry. It looks thick and nourished. the best its ever looked and other people are noticing too… but its not just about my hair…. I feel better in general. This is the first blend bought. I’ve since bought Trinity, Nebula and have received samples of seven sages and super nova.. all amazing. I never want to be without these products. I love how I feel on them… sometimes I fast with them… but even when I don’t. I still feel the positive effects of the blends.
S.R. (verified owner) –
Where to begin…. I have fasted, both wet and dry, for years prior to coming across Interstellar Blends over a year ago. Like most, I lurked and read testimonial after testimonial wondering if there was actually truth to what I was reading. I am a great lover of science, so Gavin’s website was a treasure trove for me. There was article upon article convincing me that the testimonials I was reading could actually be true.
I reached out to Gavin and he quickly responded to my inquiry. That alone spoke volumes about his integrity. I then placed an order and received it within a couple of days. The packaging was SUPERB! It was so beautiful that I didn’t even want to open it out of fear of messing it up, but boy am I glad I did.
I was never a fan of coffee because it gave me the jitters, but I still followed the instructions. The blends tasted horrible so I quickly became grateful for the coffee that helped mask the taste. Within a few minutes, I started to feel different. There was something happening, but I wasn’t sure what or how to explain it. I was feeling an “opening” going on in my chest and head. Soon, my body began to crave the blends, nasty tasting and all!
I have already tried most blends, but I want to focus on Super Tonic Hair and Pine Pollen. As I mentioned previously, I was no stranger to fasting; and as most would know, that usually means hairloss. My hair had become so thin and brittle that I felt it was a lost cause. I began taking Super Tonic Hair and Pine Pollen, and within a month, I began to get compliments on my hair’s fullness and shine. I no longer had to choose between fasting and my hair. With Interstellar Blends, I can have it all!!
Thank you Gavin for sharing your wealth of knowledge and your products with the world. You and your whole team are positively impacting and changing lives worldwide!!!
Kevin (verified owner) –
After I noticed my hair thinned out really quick I decided to order super tonic hair, I was like: nothing to lose right, so why not try it out? After a week I immediately noticed my hair didn’t fall off easily anymore and I’m just a week in!
Also I noticed that beside growing back thicker and less falling out that the quality became better. Also it tastes really good! Like a light version of coffee which makes it part of my morning routine.
Can’t wait for what the coming months will bring, but I sure do now that I will order now right away another one.
Bal Purewal (verified owner) –
I feel very blessed and grateful that i started taking super hair tonic 4 weeks ago. To be honest – it was not my hair that was a priority for me as i have other issues to deal with. However , I purchased the combo pack of blends ( thought that was my best way forward to take all the blends as per Gavins 22:2 protocol . Like i said at the beginning – my hair was not really my main priority for now – main reason being i fell out with my hair 30 years ago when i got my first grey hair (i was 13 years old) – i knew this wa genetic as my family members also started going grey as children – by the time i was 27 years old i succumbed to dying my hair ( so next 24 years was a big commitment to dying my hair every 4 weeks) , in that time – i knew the hair dyes were no good – toxic and carcinogenic – i kept dying my hair as could not leave my house if i saw one white hair!!!! Then we had the first lockdown March 2020- and thats the ush i needed to stop dying my hair – however – i felt that the damage had already been done to my hair – it was thin – lacked lustre and i had over the years developed 2 very very thin (almost bald ) patches on my hairline (above my temples ) which i felt i had to accept as never grew back- Now back to the super tonic hair – started taking 4 weeks ago – one quarter teaspoon once a day only – and my hair seems to have thickened considerably – the bald patches do not look as bald anymore and I’m feeling so much confident with my hair and not afraid to go out . At this time of writing this review- i personally not sure if my grey hairs are reducing and my natural colour coming back ( it appears like it is) – i will write another update in couple months time . Needless to say i have now purchased a 100g bag – without batting an eyelid – i would advise anyone who cares about their hair – STOP GOING TO THE HAIR DRESSERS – DONT ALLOW CARCINIGENS IN HAIR DYES-AND HAIR PRODUCTS ANYWHERE NEAR YOUR SCALP AND HAIR – THE MONEY YOU SAVE NOT GOING TO HAIRDRESSERS WILL BE MORE THAN YOU NEED FOR THE SUPERTONIC HAIR – you will never go back – for the first time in my life – i love my hair – even the greys – i call myself the OCEAN PRINCESS
Suzanne Kici (verified owner) –
I have been using this product for approximately 12 months and I cannot believe how healthy my hair is. My stylist said that it is so much thicker and has more volume and is not dry like it used to be. I absolutely love this product!
Valerie Cade (verified owner) –
As a woman who is 53 with thinning hair due to menopause hormonal issues, Super Hair Tonic truly restored my thinning hair to the fullness I’d experienced before ~ and within on week. My mistake was only ordering the sample; I now realize ordering the full package is the key as this blend is a staple for me moving forward (along with peel and spice for every day muscle aches and pains; these are gone, trinity for focus and motivation and autophagy for controlling hunger as I continue to lose weight and not have any cravings). Super Hair Tonic exceeded my expectations!
Harry Aplin (verified owner) –
I’ve been using this product for the last two weeks and I’m very impressed.. it took about 5 days for me to notice a change, with the strands of my hair looking much thicker and healthy..after a couple of weeks I noticed my eye lashes got really long too! I’ve never seen them so long before!
I used to suffer from an itchy scalp before using this product but now it feels so good to be free of that, super happy with the results and will be buying more for sure, also keen to try out some of the other products!
Thankyou Gavin!
Patricia (verified owner) –
I’ve been taking the blends for over a year. LET ME TELL YOU, these are by far the BEST thing I stumbled upon. I have always been into alternative medicine and am never willing to take what big Pharma is pushing on me. I am a retired Registered Nurse and have pushed back against Pharma my entire career…weird right but my eyes were opened.
I have suffered with arthritis, pain, low energy but feeling of impending doom, thinning hair, and high blood pressure. Let me tell you….I tried many supplements to try and reverse these symptoms and nothing worked. Again by the Grace of God, I came across Gavin and his life saving blends. What a game changer!!
Here’s my results. By taking Peel and Spice for Arthritis, I have function back in my wrists and pain is under control. Low Energy and impending doom? GONE with NEBULA and TRINITY…I have never felt so calm and let things pass in my entire life! I use to be high strung and everything effected me. After having what I think was COVID..(never got tested) I started loosing my hair. How disturbing to me. I have been taking the hair blend and is thankfully growing back. Last but not least, EKG has brought my blood pressure into normal range. At every annual physical, my blood pressure was so high sometimes they didn’t want me to leave the clinic. This is a game changer for me. So happy I’m not in the critical zone anymore.
I could go on and on about the blends. Purge, Glucose Blocker, Anti Adipogenic, Thermo, and Autophagy for loosing weight…OFF THE CHARTS. I lost 10 pounds and have kept it off for over a year.
This is getting long so just try them….you’ll never go back! Thank You Gavin so much for your life saving and beautiful blends. They are filled with Love.
Kristina (verified owner) –
I have been taking super hair formula for 2 months now. I bought it to mainly help with gray hairs. I am 33 but started getting grays in my late 20’s. My hair has also been thinning and not growing past a particular length. I did not notice results the first 2 weeks, but kept taking the blend since I had it I figured I might as well finish it off. In the 3rd week, I noticed this bump/wart I had at the nape of my hairline disappeared. It had been there for as long as I can remember. I was shocked to run my finger where it used to be and feel smooth skin. I felt encouraged.
I have been wearing tape-in extensions for about 2 years to give myself the appearance of longer, thicker hair. 6 weeks in of taking the blend, I had a routine hair appointment to remove, clean my extensions, re-install them, and get my roots colored. As my hair stylist was removing them, she commented on how much new growth she was seeing. When she finally removed them all, we were both shocked to see my actual hair had grown as long as the extensions!!!! This has never happened before! My hair was also so much thicker. She only installed half as many as I used to get, and for my next appointment in a couple weeks, I am going to remove them altogether.
I have not yet noticed an improvement with the grays, but I think I will be able to analyze results better once I take my extensions out and take a break from hair coloring. I think it may just take longer to see that change, but I feel encouraged because of the amazing results in length and thickness. I will update after a few more months on the improvement of grays! This is a wonderful blend but it takes a few weeks to see the results. Do not be discouraged if you don’t see a change right away! Stick with it and be consistent.
Diana Ricco (verified owner) –
Super Tonic Hair is a life saver!!! I was starting to lose my hair in clumps after I had covid. Not a single remedy worked to stop the hair loss, let alone grow it back. I was super thinned out and I couldnt even pass a comb through my hair without losing a bunch of it. Enter this blend!
Within a month of taking it, i was sprouting fresh new hairs all over my head. My existing hair felt stronger and less brittle, AND i havent seen any random strands of grey hair since I started taking this blend. I am a repeat customer already and its been about a year now. My hair is thicker than before. It is stronger than before. It feels and looks way healthier than before.
Even my hairdressers are shocked by the drastic change in thickness and hair growth that theyve all been asking me for the name of this blend. They tell me they have NEVER seen anything like the results theyve seen on me, and they see people’s hair every day. It is truly life changing. It brought me from being stressed and depressed to being confident agian. I dont think I will ever not buy this blend for as long as I live. Thank you, Gavin.
Konstantin K (verified owner) –
One month ago I placed my first order and boy was I not disappointed! First of all, I can feel all of them, each has a very pronounced effect. Supernova makes me super focused and alert, gainz significantly increases my muscle mass and strength, the rest just make me feel better in general. I’ve been experimenting with all sorts of different supplements and practices over the last 2-3 years but I have never felt such prominent effects.
Although, in this review I’d like to particularly highlight Super Hair. I’ve been dealing with slowly receding hairline since I was 20. I started fighting it right when it started, doing all sorts of things from natural toos like microneedling and rosemary oil to more kind of pahrma things like minoxidil. Last few years even though I’ve been doing a lot, the receding hairline started to win the battle. I’ve been seriously thinking about doing hair transplant in the next couple of months.
Hard to put in words how amazed I was with the results I got from Super Hair. Never have I ever seen such a FAST and PRONOUNCED effect. One week in I could see new hairs growing and old ones getting thicker. No other hair solution or compound works that fast. Minoxidil takes around 8 weeks to see the first tiny results. Hats off to Gavin for creating such a powerful and natural solution. 👏👏👏
Lori Cramer –
I have been using this blend a little over a month, so I can’t give a full review as yet but I am liking what I see. Fuller thicker hair ..i definitely see a difference from before. So im pretty excited to keep using and see more results. I am definitely excited to try other blends.
Jennifer W –
It’s funny how you get used to feeling a certain way so that’s just what you accept of yourself. Or you aren’t aware that something is wrong with you until you feel better. I didn’t realize how exhausted I get during the day. I didn’t realize how low energy I am. I just go through my day and anxiously await a proper time to go to bed. Or…. what’s it feel like not to have a headache? I don’t get migraine headaches, it’s just from not sleeping. I can expect a headache at least four times a week. Stomach aches from poor digestion are awful too. Just overall feeling good… really? Yes!!! Yes!!! Yesssss!!!!
Interstellar Blends to the rescue. So I have several of the blends in sample size. I’ve actually had them quite a while and I would take some here, take some there. I really got serious about it this past week and I have taken them at least twice daily and sometimes three times daily. WOW is all I can say. The blends certainly have a way of pointing out just how poorly I felt by making me feel amazing!!
I have been taking a concoction of plush, nebula, super hair, spice, niagra, super nova, and several more. The first thing I noticed was that I can actually breathe through my nose. I have absolutely no congestion. I suffer with seasonal allergies and allergens from indoors. I work in an over 100 year old building and there’s stuff everywhere in there. I have zero congestion and zero allergy symptoms.
Energy? Ha!!! I’m freaking annoying because I have so much energy. Sleep and blends!! No more headaches. Not a single one this week. An overall mellow mood and I’m nice. I’m so, so nice now!!! LOL Digestion issues gone!! When I take steps to feel better I don’t want to put the crap in my body so I’m eating a better diet too. I’m fasting and taking the blends with coffee or tea.
The blends go above and beyond what they’re intended to help you with. All this and I haven’t even tried the blends for sleep. Since I’m headed into the weekend I will give that a shot…just so I can adjust the amount so I will get up in the morning for work when Monday rolls around.
The Interstellar Blend website is so amazing. I love the updated “choose your own symptom” scrolling technology provided. It makes choosing blends so convenient.
Thank you Gavin and Interstellar Blends. I haven’t felt this good in a very long time…. Possibly ever!!