
HELICO (Helicobacter Pylori Destroyer)
November 8, 2019
SENOLYTIC (Zombie Cell Killer)
April 2, 2020ANTI-ADIPOGENIC (Adipocyte Differentiation Inhibitor)
$275.00
introducing
interstellar blend
ANTI
ADIPOGENIC
200:1 CONCENTRATION







Adipogenesis refers to the process of formation of adipose tissue. It is a multistep process starting with clonal expansion of mesenchymal cells and the Differentiation of these mesenchymal cells into Pre-Adipocytes and finally into mature Adipocytes.
Herbal Remedies against Adipogenesis
Many phytochemicals and herbal extracts show Inhibitory effects on Adipogenesis in cell models and mouse models. However, lack of clinical evidence and epidemiological data to support their roles in Inhibiting Adipogenesis hindered their development to become an Anti-Obesity therapeutic agent. Furthermore, many of these phytochemicals have low bioavailability. Chemical structural modification or targeted delivery system may help to increase their Anti-Adipogenesis or Anti-Obesity efficacies. Nevertheless, many of these phytochemicals are abundantly found in our daily food, or they are the herbs for cooking or seasoning. For example, resveratrol is rich in red wine and grapes; genistein is rich in soy; quercetin in apples and onions, and curcumin is a well-known component of the cook seasoning. Therefore, a healthy diet can definitely help us to increase the uptake of these phytochemicals in our daily life. Moreover, combination of phytochemicals or herbal extracts may act synergistically to Inhibit Adipogenesis.
Indeed, Obesity is not simply an excess accumulation of white Adipose Tissue but is usually associated with insulin resistance and an increased production of metabolic hormones coupled with chronic low-grade state of Inflammation . To effectively Reduce Obesity , a holistic strategy with the consumption of phytochemicals or herbal extracts can be designed not only to Reduce Adipose Tissue Mass, but also increase thermogenic energy expenditure, improve insulin sensitivity, Reduce plasma lipids which can help to Reduce the Obesity -associated dyslipidemia and other comorbid conditions.
Obesity is a global health problem characterized as an increase in the Mass of Adipose Tissue . Adipogenesis is one of the key pathways that increases the Mass of Adipose Tissue , by which preAdipocyte s mature into Adipocytes through cell Differentiation . Peroxisome proliferator-activated receptor γ (PPARγ ), the chief regulator of Adipogenesis , has been acutely investigated as a molecular target for natural products in the development of anti-Obesity treatments. In this review, the regulation of PPARγ expression by natural products through Inhibition of CCAAT/enhancer-binding protein β (C/EBPβ) and the farnesoid X receptor (FXR), increased expression of GATA-2 and GATA-3 and activation of the Wnt/β-catenin pathway were analyzed.
Furthermore, the regulation of PPARγ transcriptional activity associated with natural products through the antagonism of PPARγ and activation of Sirtuin 1 (Sirt1) and AMP-activated protein kinase (AMPK) were discussed. Lastly, regulation of mitogen-activated protein kinase (MAPK) by natural products, which might regulate both PPARγ expression and PPARγ transcriptional activity, was summarized. Understanding the role natural products play, as well as the mechanisms behind their regulation of PPARγ activity is critical for future research into their therapeutic potential for fighting Obesity.
Effects of Flavonoids and Phenolic Acids on the Inhibition of Adipogenesis in 3T3-L1 Adipocytes
Obesity has become a global epidemic in both developed and developing countries, and it is a significant risk factor for various diseases such as Diabetes , cancer, heart disease, and hypertension. In the present study, the effect of naturally occurring Antioxidants (Flavonoids and Phenolic Acids ) on the Inhibition of Adipogenesis in 3T3-L1 Adipocytes was investigated. The results showed that o-coumaric acid and rutin had the highest Inhibition on intracellular triglyceride (61.3 and 83.0%, respectively) among 15 Phenolic Acids and 6 Flavonoids tested. However, the oil red o stained material (OROSM) showed that cell number in 3T3-L1 Adipocytes was not influenced by those compounds.
For glycerol-3-phosphate dehydrogenase (GPDH) activity, the data indicated that o-coumaric acid and rutin had the highest Inhibition on GPDH activity (54.2 and 66.8%, respectively) among the compounds tested. o-Coumaric acid and rutin also Inhibited the expression of PPARγ , C/EBPα and leptin and then up-regulated expression of adiponectin at the protein level. Some naturally occurring Antioxidants efficiently suppressed Adipogenesis in 3T3-L1 Adipocytes. These results suggest that o-coumaric acid and rutin targeted for Adipocyte functions could be effective in improving the symptoms of Metabolic Syndrome.
INGREDIENTS & science
açai (Euterpe oleracea Martius)
The anti-lipidaemic and anti-inflammatory effects of açai Polyphenols in 3T3-L1 mouse Adipocytes were investigated. Pre-Adipocytes were differentiated with and without açai-Polyphenols at concentrations of 2.5, 5 and 10 µg gallic acid equivalents (GAE)/mL. Results showed that açai Polyphenols Reduce the accumulation of intracellular lipids in differentiated Adipocytes in a dose-dependent manner and downregulated PPARγ 2. The gene-expression of Adipogenic transcription factors C/EBPα, C/ebpβ, Klf5 and Srebp1c was decreased.
This was accompanied by a reduction of Adipogenic genes, including aP2, LPL, FATP1 and FAS, leptin and total PAI and an increase of adiponectin. Additionally, açai Polyphenols protected cells against the production of ROS and decreased the expression of mRNA and protein levels of pro-inflammatory cytokines when 3T3-L1 cells were challenged with TNF-α. Thus, these results indicate that açai Polyphenols may be useful in the prevention of Adipogenesis, oxidative stress and Inflammation.

In this study, we observed that LEAO decreased the accumulation of lipid droplets during Adipogenesis and down-regulated the expression of key Adipogenic transcription factors such as peroxisome proliferator-activated receptor γ (PPAR γ) and CCAAT/enhancer binding protein α (C/EBP α). In addition, LEAO inactivated PI3K/Akt signaling and its downstream factors that promote Adipogenesis by inducing the expression of PPAR γ. LEAO also activated β-catenin signaling, which prevents the Adipogenic program by suppressing the expression of PPAR γ. Therefore, we found that treatment with LEAO is effective for attenuating Adipogenesis in 3T3-L1 cells . Consequently, these findings suggest that LEAO has the potential to be used as a therapeutic agent for preventing Obesity.

The present study investigated the AntiObesity effect of Achyranthes bidentata Blume root water extract in a 3T3-L1 Adipocyte Differentiation model and rats fed with a high-fat diet. To investigate the effect of Achyranthes bidentata Blume on Adipogenesis in vitro, differentiating 3T3-L1 cells in Adipocyte -induction media were treated every two days with Achyranthes bidentata Blume at various concentrations (1 to 25 μg/mL) for eight days. We found that Achyranthes bidentata Blume root Inhibited 3T3-L1 Adipocyte Differentiation without affecting cell viability and Western blot analysis revealed that phospho-Akt expression was markedly decreased, whereas there was no significant change in perilipin expression.

Acorn (Quercus acutissima CARR.) is a nut from the Fagaceae family that has been used in traditional medicine for many years. However, shells from acorns are regarded as a by-product and are mostly discarded. Anti-Adipogenic activities of acorn shells were investigated using 3T3-L1 cells and methanol shell extracts (AE-M). AE-M demonstrated Cu2+-chelation activities and anti-oxidant activities via reduction of oxidative stress levels induced using AAPH. Six days after Adipocyte Differentiation, 50 and 100 μg/mL AE-M completely suppressed 3T3-L1 Adipogenesis and the Anti-Adipogenic effect was stronger than for the positive control 50 μM quercetin.
Treatment with AE-M in 3T3-L1 cells Reduced mRNA expression levels of Adipogenic genes. AE-M-Inhibition was found in Pre-Adipogenic, early, and intermediate stages of Adipogenesis in 3T3-L1 cells. The Wnt/β-catenin signaling pathway is required for AE-M-Inhibition of 3T3-L1 Adipogenesis.
Adenanthin (isodon adenantha)
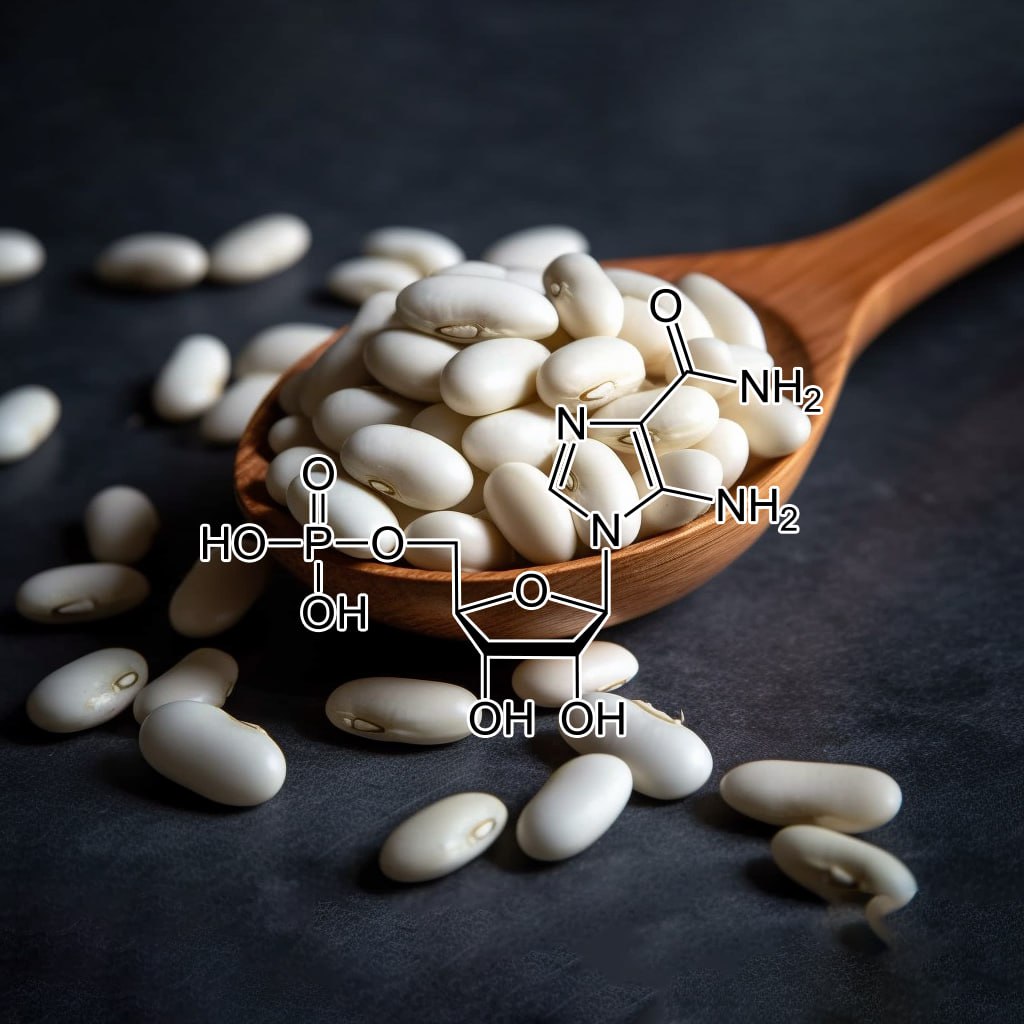
The Effects of AICAR on Adipocyte Differentiation of 3T3-L1 cells
The AMP-activated protein kinase (AMPK) activator, 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), has been found to Inhibit the Differentiation of 3T3-L1 Adipocytes, if added at an early phase of Differentiation. AICAR blocks the expression of the late Adipogenic markers, fatty acid synthase and acetyl-CoA carboxylase, and of the transcription factors, C/EBPα and PPARγ. It also Inhibits early clonal expansion of Pre-Adipocytes, prevents the fall in C/EBPβ expression during the intermediate stage of Differentiation and Inhibits the late phase expression of CHOP-10, an antagonist of C/EBPβ. These data suggest a possible Inhibitory role for AMPK in the process of adipose Differentiation and suggest that AMPK might be a target to block Adipogenesis.
Results: AICAR blocked Adipogenic conversion in 3T3L1 cells along with significant decrease in the neutral lipid content by downregulating several Adipocyte -specific transcription factors including peroxisome proliferators-activated receptor γ (PPARγ), C/EBPα and ADD1/SREBP1, which are critical for Adipogenesis in vitro. Moreover, intraperitoneal administration of AICAR (0.5 mg g/body weight) to mice fed with high-fat diet (60% kcal% fat) to induce DIO, significantly blocked the body Weight Gain and total content of epididymal fat in these mice over a period of 6 weeks. AICAR treatment also restored normal adipokine levels and resulted in significant improvement in glucose tolerance and insulin sensitivity. The reduction in Adipose Tissue content in AICAR treated DIO mice was due to reduction in lipid accumulation in the pre-existing Adipocytes. However, no change was observed in the expression of PPARγ, C/EBPα and ADD1/SREBP1 transcription factors in vivo though PGC1α expression was significantly induced.
Conclusion: Our study demonstrates that AICAR treatment significantly attenuates adipoctye Differentiation in vitro. However, its administration restricted the body weight, epididymal fat content and normalized metabolic alteration mediated by diet induced Obesity in mice. Increase in phosphorylation of ACC and AMPKα during Adipocyte Differentiation and AMPK activity in epididymal Adipose Tissue in DIO mice raises doubts about the involvement of AMPK in this process.
Ajoene Sulfur (Allium sativum)
Allium hookeri root
Anti-Adipogenic and AntiDiabetic activities of Allium hookeri root water extracts (ARW) were assessed. Oil Red O staining showed that treatment with ARW caused a dose-dependent reduction in lipid accumulation. ARW was also involved in Adipocyte Lipolysis via LPL activity, and in the concentration of glycerol in a culture medium. On the basis of the concentration of adipokines following ARW treatment, ARW appeared to Inhibit expression of PPAR-γ, to Reduce concentrations of leptin and resistin, to increase the concentration of adiponectin, and to Inhibit lipid accumulation. ARW modulated adipokine expression associated with insulin resistance and sensitivity. 3T3-L1 Adipocytes treated with ARW showed increased GLUT-4 expression with increased glucose uptake into Adipocytes. ARW showed effectiveness for improvement of Diabetic conditions.
Andrographolide (Andrographis paniculata)
Andrographolide suppressed cyclin A, cyclin E, and CDK2 expression and impaired the progression of mitotic clonal expansion (MCE) by arresting the cell cycle at the Go/G1 phase. Taken together, these results indicate that andrographolide has a potent Anti-Obesity action by Inhibiting PKA-CREB-mediated C/EBPβ expression as well as C/EBPβ transcriptional activity, which halts MCE progression and attenuates C/EBPα and PPARγ expression.
Anthocyanins
Regulation of Adipocyte Function by Anthocyanins ; Possibility of Preventing the Metabolic Syndrome
Obesity is defined as the accumulation of excess Adipose Tissue resulting from various metabolic disorders. Adipocyte dysfunction is strongly associated with the development of Obesity and insulin resistance. Metabolic Syndrome is characterized by a group of metabolic risk factors in one person. Abdominal Obesity and Adipocyte dysfunction play an important role in the development of this syndrome.
Anthocyanins are used as a food coloring, and they are widely distributed in human diets including berries, suggesting that large amounts of Anthocyanins are ingested from plant-based foods. This study shows that Anthocyanins have a significant potency of AntiObesity and ameliorate Adipocyte function in in vitro and in vivo systems and also that they have important implications for preventing Metabolic Syndrome.
Anthocyanins markedly Reduced gene and protein expression levels of lipogenic transcription factors such as liver X receptor α, sterol regulatory element-binding protein-1c, peroxisome proliferators-activated receptor-γ, and CCAAT enhancer-binding protein-α. In addition, the target gene and protein expression of these lipogenic transcription factors such as fatty acid synthase, stearoyl-CoA desaturase-1, and acetyl-CoA carboxylase α were markedly suppressed by Anthocyanins. Thus, Anthocyanins suppress lipid accumulation in Adipocytes due to broad Inhibition of the transcription factors regulating lipogenesis. This may partially explain the mechanism by which Anthocyanins exert their Anti-Obesity effect.
Antofine Alkaloid
Antrodia cinnamomea
Apigetrin (apigenin-7-O-glucoside)
Results: Our results showed that apigterin treatment Inhibited significantly lipid accumulation without effect on cell viability at 100 μM, and it exerted the Anti-Adipogenic effect during the early stages of Differentiation. Flow cytometry analysis showed that apigenin-7-O-glucoside (Ap7G) Inhibited cell proliferation during mitotic clonal expansion and caused cell cycle delay. Quantitative PCR analysis revealed that the mRNA levels of C/EBP-α, PPAR-γ, SREBP-1c and FAS were suppressed after apigetrin treatment at 100 μM. Moreover, the mRNA level of pro-inflammatory genes (TNF-α and IL-6) were suppressed after apigterin treatment, at high concentration Preadipocyte cells.
Conclusion: Taken together, these results indicated that apigenin-7-O-glucoside Inhibits Adipogenesis of 3T3-L1 preadipocytes at early stage of Adipogenesis.
Arctigenin

Results: Arctiin treatment to 3T3-L1 Pre-Adipocytes markedly decreased Adipogenesis in a dose-dependent manner. The arctiin treatment significantly decreased the protein levels of the key Adipogenic regulators PPARγ and C/EBPα, and also significantly Inhibited the expression of SREBP-1c, fatty acid synthase, fatty acid-binding protein and lipoprotein lipase. Also, arctiin greatly increased the phosphorylation of AMP-activated protein kinase (AMPK) and its downstream target phosphorylated-acetyl CoA carboxylase. Furthermore, administration of arctiin significantly decreased the body weight in obese mice fed with the high-fat diet. The epididymal, perirenal or total visceral Adipose Tissue weights of mice were all significantly lower in the HF + AC than in the HF. Arctiin administration also decreased the sizes of lipid droplets in the epididymal Adipose Tissue.
Conclusions: Arctiin Inhibited Adipogenesis in 3T3-L1 Adipocytes through the Inhibition of PPARγ and C/EBPα and the activation of AMPK signaling pathways. These findings suggest that arctiin has a potential benefit in preventing Obesity.
Aristolochia Manshuriensis
Oral administration of AMK (62.5 mg/kg/day) significantly decreased the fat tissue weight, total cholesterol (TC), and low density lipoprotein-cholesterol (LDL-C) concentration in the blood. The results of this study suggested that AMK Inhibited lipid accumulation by the down-regulation of the major transcription factors of the adipogensis pathway including PPAR-γ and C/EBP-α through regulation of Akt pathway and ERK 1/2 pathway in 3T3-L1 Adipocytes and HFD-induced Obesity mice, and AA may be main act in Inhibitory effects of AMK during Adipocyte Differentiation.
Herbal Remedies against Adipogenesis
Aristolochia manshuriensis Kom is a traditional medicinal herb used for treatment of arthritis, rheumatism, hepatitis. Its extract Inhibited Adipocyte Differentiation by regulating ERK1/2 and Akt pathway. Besides, expressions of FAS, LPL and aP2 were significantly Reduced by the extract treatment during Adipogenesis.
Aristotelia chilensis
Interest in berries from South America has increased due to their potential health benefits. The objective of this study was to characterize the Anthocyanins and proanthocyanidins of Vaccinium floribundum and Aristotelia chilensis, total phenolics, and antioxidant capacity and to evaluate, in vitro, the ability of their phenolic extracts to Reduce Adipogenesis and lipid accumulation in 3T3-L1 Adipocytes. The anti-inflammatory property of these extracts on RAW 264.7 macrophages was also investigated. Antioxidant capacity, measured as oxygen radical scavenging capacity and expressed as Trolox equivalents, was higher in the berries of A. chilensis. Phenolic extracts Inhibited lipid accumulation by 4.0−10.8% when Adipocytes were treated at maturity and by 5.9−37.9% when treated throughout Differentiation.
Furthermore, a proanthocyanidin-enriched fraction from V. floribundum significantly increased Pref-1 expression in preadipocytes. Phenolic extracts decreased the production of nitric oxide (3.7−25.5%) and prostaglandin E2 (9.1−89.1%) and the expression of inducible nitric oxide synthase (9.8−61.8%) and cycloxygenase-2 (16.6−62.0%) in lipopolysaccharide-stimulated RAW 264.7 macrophages. V. floribundum and A. chilensisphytochemicals limit Adipogenesis and inflammatory pathways in vitro, warranting further in vivo studies.

Obesity has increased continuously in western countries during the last several decades and recently become a problem in developing countries. Currently, Anti-Obesity drugs originating from natural products are being investigated for their potential to overcome adverse effects associated with chemical drugs. Artemisinic acid, which was isolated from the well-known anti-malaria herb Artemisia annua (AA) L., was recently shown to possess Anti-Adipogenic effects in vitro. However, the Anti-Adipogenic effects of AA in animal models have not yet been investigated.
Therefore, we conducted daily oral administration with AA water extract in a diet-induced Obesity animal model and treated 3T3-L1 cells with AA to confirm the Anti-Adipogenic effects in the related protein expressions. We then evaluated the physiology, Adipose Tissue histology and mRNA expressions of many related genes. Inhibition of Adipogenesis by the AA water extract was observed in vitro. In the animal model, Weight Gain was significantly lower in the AA treated group, but there were no changes in food intake volume or calories. Reductions in lipid droplet size and mRNA expression associated with Adipogenesis were also observed in animal epididymal fat. This study is the first to report that AA has an anti-obese effects in vivo.
Aster yomena
The leaves of Aster yomena (Kitam.) Honda have long been used as a traditional herb for treating disorders including coughs, asthma, and insect bites. According to recent studies, A. yomena leaf extracts have several pharmacological properties, including anti-inflammatory, antioxidant, and anti-asthmatic activities. However, little information is available regarding their Anti-Obesity effect. In this study, we investigated the Inhibitory effect of the ethanol extracts of A. yomena leaves (EEAY) on Adipocyte Differentiation and Adipogenesis using 3T3-L1 preadipocytes. When 3T3-L1 preadipocytes were treated with various concentrations of EEAY (ranging from non-toxic), the number of lipid droplets, lipid content, and triglyceride production, the typical characteristics of Adipocytes, were suppressed in a concentration-dependent manner.
During this process, EEAY significantly Reduced the expression of Adipogenic transcription factors, including peroxisome proliferator-activated receptor-γ, CCAAT/enhancer-binding protein α and β, and sterol regulatory element-binding protein-1c. In addition, EEAY was also found to potently Inhibit the expression of Adipocyte-specific genes, including Adipocyte fatty acid-binding protein and leptin. In particular, EEAY treatment effectively enhanced the activation of the AMP-activated protein kinase (AMPK) signaling pathway; however, the co-treatment with compound C, an Inhibit or of AMPK, significantly restored the EEAY-induced Inhibition of Pro-Adipogenic transcription factors and Adipocyte-specific genes. These results indicate that EEAY may exert an Anti-Obesity effect by controlling the AMPK signaling pathway, suggesting that the leaf extract of A. yomena may be a potential Anti-Obesity agent.
Astilbe chinensis
Astilbe chinensis Franch. et Savat. (AC) has been used in traditional medicine for the treatment of chronic bronchitis, arthralgia, and gastralgia. In this study, we investigated the AntiObesity effect of AC extract on 3T3-L1 preadipocytes and high-fat-diet-fed C57BL/6N obese mice. We found that AC extracts dramatically decreased the lipid content of 3T3-L1 cells in a concentration-dependent manner without cytotoxicity. The action mechanism of AC extract was demonstrated to be the Inhibition of lipid accumulation and dose-dependent decrease in the expression of CCAAT/enhancer-binding protein α (C/EBPα), peroxisome proliferator-activated receptor-γ (PPAR-γ), and sterol regulatory element-binding protein 1 (SREBP1).
Furthermore, AC extract increased the mitochondrial phosphorylation of AMP-activated protein kinase (AMPK) and acetyl-CoA carboxylase (ACC), mitochondrial biogenesis, and Lipolysis -related factors. In amice model of high-fat-diet-induced Obesity, the mice administered AC extract experienced significant decrease of 64% in Weight Gain, 55% in insulin resistance index, 22% in plasma triglycerides (TG), 56% in total cholesterol (TC), and 21% in nonesterified fatty acid (NEFA) levels compared with those in the high-fat diet-fed control mice. Collectively, these results indicated that AC extract exerted antiobesogenic activity through the modulation of the AMPK signaling pathway, Inhibition of Adipogenesis, decreased lipid content, and Reduced Adipocyte size.
Astragalin (3-O-glucoside kaempferol)
Astilbe chinensis Franch. et Savat. (AC) has been used in traditional medicine for the treatment of chronic bronchitis, arthralgia, and gastralgia. In this study, we investigated the AntiObesity effect of AC extract on 3T3-L1 preadipocytes and high-fat-diet-fed C57BL/6N obese mice. We found that AC extracts dramatically decreased the lipid content of 3T3-L1 cells in a concentration-dependent manner without cytotoxicity. The action mechanism of AC extract was demonstrated to be the Inhibition of lipid accumulation and dose-dependent decrease in the expression of CCAAT/enhancer-binding protein α (C/EBPα), peroxisome proliferator-activated receptor-γ (PPAR-γ), and sterol regulatory element-binding protein 1 (SREBP1).
Furthermore, AC extract increased the mitochondrial phosphorylation of AMP-activated protein kinase (AMPK) and acetyl-CoA carboxylase (ACC), mitochondrial biogenesis, and Lipolysis-related factors. In amice model of high-fat-diet-induced Obesity, the mice administered AC extract experienced significant decrease of 64% in Weight Gain, 55% in insulin resistance index, 22% in plasma triglycerides (TG), 56% in total cholesterol (TC), and 21% in nonesterified fatty acid (NEFA) levels compared with those in the high-fat diet-fed control mice.
Collectively, these results indicated that AC extract exerted antiobesogenic activity through the modulation of the AMPK signaling pathway, Inhibition of Adipogenesis, decreased lipid content, and Reduced Adipocyte size.
Averrhoa carambola
Obesity is associated with an increased risk of many chronic diseases. Recently, a growing body of evidence has shown that phytochemicals may Inhibit Adipogenesis and Obesity. In this study, we report for the first time, the ability of Averrhoa carambola L. peel extract commonly known as star fruit (SFP) to effectively suppress Adipocyte Differentiation in 3T3-L1 preadipocytes and therefore, address it as a potential candidate to treat Obesity and its related diseases. (−)-Epicatechin was identified as a bioactive compound likely responsible for this suppression.
As the genetic expression studies revealed that the Adipogenic activity of SFP extract was due to the simultaneous downregulation of the C/EBPα and PPARγ as well as the upregulation of PPARα receptor genes, a detailed computational docking study was also elucidated to reveal the likely binding mode of (−)-epicatechin to the receptor of interest, accounting for the likely mechanism that results in the overall suppression of Adipocyte Differentiation.
Avicularin (Guava leaves)
Avicularin (quercetin-3-O-α-l-arabinofuranoside) is a plant flavonoid and a quercetin glycoside. In this study, we found that avicularin suppressed the accumulation of intracellular lipids through repression of glucose transporter 4 (GLUT4)-mediated glucose uptake in mouse adipocytic 3T3-L1 cells. Avicularin was highly purified (purity of more than at least 99%) from Taxillus kaempferi (DC.) Danser (Loranthaceae) by high-performance liquid chromatography, and its structure was determined by nuclear magnetic resonance and Mass spectrometry. Avicularin decreased the intracellular triglyceride level along with a reduction in the expression of Adipogenic genes such as peroxisome proliferator-activated receptor γ, CCAAT/enhancer-binding protein (C/EBP) α, and aP2 (fatty acid-binding protein 4).
In contrast, avicularin did not affect the expression of lipogenic and lipolytic genes. Interestingly, the expression of the GLUT4 gene was significantly suppressed in an avicularin-concentration-dependent manner. Moreover, the binding of C/EBPα to the promoter region of the GLUT4 gene was repressed by adding avicularin to the medium in 3T3-L1 cells, as demonstrated by the results of a chromatin immunoprecipitation assay. These results indicate that avicularin Inhibited the accumulation of the intracellular lipids by decreasing C/EBPα-activated GLUT4-mediated glucose uptake in Adipocytes.
Bacaba (Oenocarpus bacaba)
Bacaba phenolic extract attenuates Adipogenesis by down-regulating PPARγ and C/EBPα in 3T3-L1 cells
Bacaba (Oenocarpus bacaba Mart.) is a native Brazilian palm fruit with a high amount of Polyphenol ics, reported to have an apoptotic effect on cancer cells [1]. Here, we examined the effect of Bacaba phenolic extract (BPE) on Adipogenesis using 3T3-L1 preadipocytes . Proliferating and differentiating Adipocytes were incubated with BPE at 6, 12, and 24 μg of gallic acid equivalents (GAE)/ml. BPE Reduced accumulation of intracellular lipids and protein expression of Adipogenic markers including PPARγ, C/EBPα, FABP4, IR-β, and adiponectin in a dose-dependent manner during Differentiation of 3T3-L1 cells into Adipocytes.
Furthermore, lipid accumulation decreased with BPE (24 μg of GAE/ml) during the early stage of mitotic clonal expansion (Days 0–2). In contrast, the Inhibition of protein expression of Adipogenic markers needed a longer duration (Days 0–4, 0–7, 2–7) of BPE incubation. These results suggest that BPE Inhibits adipogenisis in vitro via targeting transcriptional factors during the early and middle stages of Differentiation.
Baicalin (Scutellaria baicalensis)
In this study, the AntiObesity effects of baicalin, 5,6‐dihydroxyflavone‐7‐glucuronic acid, were characterized using an in vitro system of Adipogenesis, i.e. fat cell formation. Baicalin‐treatment of 3T3‐L1 preadipocytes was shown to Inhibit triglyceride accumulation and lipid droplet formation during induced Adipogenesis. Microarray analyses showed that baicalin modulated the expression of genes located in pathways such as Adipogenesis, cholesterol biosynthesis, focal adhesion and others. In the Adipogenesis pathway, treatment with baicalin significantly down‐regulated terminal Differentiation markers of Adipocytes including fatty acid binding protein 4. The effects of baicalin on the core part of the Adipogenesis pathway, however, were paradoxical; the expression levels of CCAAT/enhancer binding protein (C/EBP)β and C/EBPδ were up‐regulated, while the expression levels of the peroxisome proliferator‐activated receptor (PPAR)γ and C/EBPα were down-regulated.
The antiAdipogenic mechanisms of baicalin can be explained by its effects on the upstream part of Adipogenesis pathway; baicalin not only up‐regulates the anti Adipogenic regulators, C/EBPγ, C/EBP homologous protein and Kruppel‐like factor (KLF)2, but also down‐regulates the pro Adipogenic regulator, KLF15. The overall effects of baicalin on these upstream regulators of Adipogenesis were antiAdipogenic, resulting in the down‐regulation of downstream genes and the Inhibition of cellular Fat Accumulation.
Bamboo (Phyllostachys bambusoides)
In this study, the Inhibitory effects of bamboo leaf extracts on Adipogenesis were investigated by evaluating their activity against Adipogenic transcription factors and enzymes in 3T3-L1 Adipocytes. Bamboo leaf extracts significantly decreased triglyceride levels, and increased glycerol release in Adipocytes. Cells treated with the water extract showed significantly higher glycerol release as well as lower triglyceride contents than those treated with the ethanol extract. Both bamboo leaf extracts significantly Inhibited the expression of Adipogenic transcription factors and enzymes, such as CCAAT/enhancer-binding protein α, sterol regulatory element binding protein 1c, peroxisome proliferator-activated receptor γ, acetyl-coenzyme A carboxylase, and fatty acid synthase, and increased the expression of phospho-adenosine monophosphate-activated protein kinase.
These results show that bamboo leaf extracts Inhibited Adipogenesis in 3T3-L1 Adipocytes and that the water extract was more efficacious than the ethanol extract.
Banaba (Lagerstroemia speciosa)
The effects of extracts isolated from Lagerstroemia speciosa L. (banaba) on glucose transport and adipocyte differentiation in 3T3-L1 cells were studied. Glucose uptake–inducing activity of banaba extract (BE) was investigated in differentiated adipocytes using a radioactive assay, and the ability of BE to induce differentiation in preadipocytes was examined by Northern and Western blot analyses. The hot water BE and the banaba methanol eluent (BME) stimulated glucose uptake in 3T3-L1 adipocytes with an induction time and a dose-dependent response similar to those of insulin. Furthermore, there were no additive or synergistic effects found between BE and insulin on glucose uptake, and the glucose uptake activity of insulin could be reduced to basal levels by adding increasing amounts of BE.
Unlike insulin, BE did not induce adipocyte differentiation in the presence of 3-isobutyl-1-methylxanthine (IBMX) and dexamethasone (DEX). BE inhibited the adipocyte differentiation induced by insulin plus IBMX and DEX (IS-IBMX-DEX) of 3T3-L1 preadipocytes in a dose-dependent manner. The differences in the glucose uptake and differentiation inhibitory activities between untreated cells and those treated with BE were significant (P < 0.01). The inhibitory activity was further demonstrated by drastic reductions of peroxisome proliferator-activated receptor γ2 (PPARγ2) mRNA and glucose transporter-4 (GLUT4) protein in cells induced from preadipocytes with IS-IBMX-DEX in the presence of BE. The unique combination of a glucose uptake stimulatory activity, the absence of adipocyte differentiation activity and effective inhibition of adipocyte differentiation induced by IS-IBMX-DEX in 3T3-L1 cells suggest that BE may be useful for prevention and treatment of hyperglycemia and obesity in type II diabetics.
Benincasa hispida
Effects of Fractions from Benincasa hispida on Inhibition of Adipogenesis in 3T3-L1 preadipocytes
The effects of three fractions, hexane (BHHH), chloroform (BHHC), and ethyl acetate (BHHE), from water extract of Benincasa hispida on the underlying mechanisms of Adipogenesis were investigated in 3T3-L1 cells. Intracellular lipid droplets were stained with Oil Red O dye and quantified. Compared to control, lipid accumulation significantly decreased by 11% and 13% upon treatment with BHHC and BHHE, respectively at a concentration of 50 μg/mL. Intracellular triglyceride (TG) levels were also Reduced by 21% and 16%, respectively, at the same concentration. To determine the mechanism behind the reductions in TG content and lipid accumulation, glycerol release and expression levels of Adipogenic marker genes were measured. The levels of free glycerol released into culture medium increased by 13% and 17% upon treatment with BHHC and BHHE, respectively.
In subsequent measurements using real-time polymerization chain reaction, the mRNA levels of PPARγ, C/EBPα, and leptin significantly decreased upon treatment with BHHE (45%, 67%, and 35%) in comparison with non-treated control. These results suggest that BHHE Inhibits Adipocyte Differentiation by blocking PPARγ, C/EBPα, and leptin gene expression in 3T3-L1 cells , resulting in Reduced lipid accumulation, increased glycerol release, and intracellular triglycerides.
Berberine
This study is designed to investigate the effects of berberine (BBR) on galectin-3 (Gal-3) and the relationships to its suppressive activities on Adipocyte Differentiation , proliferation and Adiposity . Our results showed that BBR greatly suppressed the Differentiation and proliferation of mouse primary preadipocytes isolated from epididymal white Adipose Tissue (eWAT), during which the expression level of Gal-3 was down-regulated significantly. Overexpression of Gal-3 totally abolished the suppressive activities of BBR on Gal-3 expression, Preadipocyte Differentiation and proliferation. BBR Reduced Gal-3 promoter activity, destabilized its mRNA and Inhibited firefly luciferase activity of a recombinant plasmid containing the Gal-3 3′ untranslated region (UTR). Furthermore, BBR up-regulated microRNA (miRNA) let-7d expression and the suppressive activity on Gal-3 3′UTR was abolished by point mutation on the let-7d binding site. In mice fed a high-fat diet (HFD), BBR up-regulated let-7d and down-regulated Gal-3 expression in eWAT; it also suppressed Adipocyte Differentiation and proliferation and Reduced Adiposity greatly.
In summary, our study proves that BBR Inhibits the Differentiation and proliferation of Adipocytes through down-regulating Gal-3, which is closely associated with its Anti-Obesity effect. Our results may support the future clinical application of BBR for the treatment of Obesity or related diseases.
Bergamottin (Bergamot)
Bergamottin Inhibits Adipogenesis in 3T3-L1 cells and Weight Regulation in Diet-Induced Obese Mice
Obesity is a serious and increasing health problem worldwide, and the Inhibition of Adipogenesis is considered to be a potential therapeutic target for it. Bergamottin (BGM), a component of grapefruit juice, has been reported to regulate Lipolysis. However, the physiological role of BGM in Obesity has not been evaluated so far. In the present study, we investigated the effects of BGM on Obesity in 3T3-L1 cells and in mice fed a high-fat diet (HFD). BGM Inhibited Adipogenic Differentiation of 3T3-L1 cells along with a significant decrease in the lipid content by downregulating the expression of two critical Adipogenic factors, CCAAT enhancer-binding protein-alpha (C/EBP𝛼α) and peroxisome proliferator activated receptor-gamma (PPAR𝛾γ).
The expressions of target proteins such as Adipocyte fatty acid-binding protein (aP2), adiponectin, and resistin were also decreased by BGM. It activated AMP-activated protein kinase (AMPK) by increasing phosphorylation of AMPK and the downstream target acetyl-CoA carboxylase (ACC), indicating that BGM exerted its antiAdipogenic effect through AMPK activation. In the HFD-induced obese mouse model, BGM administration significantly Reduced the weight and sizes of white Adipose Tissue as well as the Weight Gain of mice fed HFD. Moreover, UCP1 and PGC1𝛼α expressions, well-known as brown Adipocyte marker genes, were higher in the BGM-treated HFD mice than that in the HFD-induced obese mice. This study suggests that BGM suppress Adipogenesis by AMPK activation in vitro and Reduce s body weight in vivo.
Betanin (Beta vulgaris)
The Inhibitory Effect of Betanin on Adipogenesis in 3T3-L1 Adipocytes
Betanin, a natural pigment that presents ubiquitously in plants, has been reported to show biological effects. However, not much is known on the effectiveness of betanin in regulating Fat Accumulation. Therefore, the aim of this study is to explore the Inhibitory effect of betanin on Adipogenesis in 3T3-L1 Adipocytes and its mechanism action. The results show betanin significantly Inhibited oil red O-stained material (OROSM) and triglyceride levels in 3T3-L1 Adipocytes, indicating betanin Inhibited lipid accumulation in 3T3-L1 Adipocytes. In addition, the peroxisome proliferator–activated receptor γ (PPARγ) expression was significantly Inhibited in the betanin-treated Adipocytes, implying that betanin suppressed the cellular PPARγ expression in 3T3-L1 Adipocytes.
Moreover, the suppression of lipid accumulation by betanin occurred by decreasing the gene expression of PPARγ, CCAAT-enhancer-binding protein α (C/EBPα) and sterol regulatory element binding protein 1c (SREBP-1c). Taken together, these findings suggest betanin may be a mediator of Adipocyte accumulation, leading to the Inhibition of lipogenesis in 3T3-L1 Adipocytes and betanin is therefore potentially useful for designing new antiAdipogenic agent.
Bisdemethoxycurcumin (Curcuma longa)
Obesity is caused by excessive accumulation of body fat and is closely related to complex metabolic diseases. Adipogenesis is a key process that is required in Adipocyte hypertrophy in the development of Obesity. Curcumin (Cur) has been reported to Inhibit Adipocyte Differentiation, but the Inhibitory effects of other curcuminoids present in turmeric, such as demethoxycurcumin (DMC) and bisdemethoxycurcumin (BDMC), on Adipogenesis have not been investigated. Here, we investigated the effects of curcuminoids on Adipogenesis and the molecular mechanisms of Adipocyte Differentiation. Among three curcuminoids, BDMC was the most effective suppressor of lipid accumulation in Adipocytes. BDMC suppressed Adipogenesis in the early stage primarily through attenuation of mitotic clonal expansion (MCE). In BDMC-treated preadipocytes, cell cycle arrest at the G0/G1phase was found after initiation of Adipogenesis and was accompanied by downregulation of cyclin A, cyclin B, p21, and mitogen-activated protein kinase (MAPK) signaling.
The protein levels of the Adipogenic transcription factors peroxisome proliferator-activated receptor (PPAR)γ and CCAAT/enhancer-binding proteins (C/EBP)α were also Reduced by BDMC treatment. Furthermore, 0.5% dietary BDMC (w/w) significantly lowered body Weight Gain and Adipose Tissue Mass in high-fat diet (HFD)-fed mice. The results of H&E staining showed that dietary BDMC Reduced hypertrophy in Adipocytes. These results demonstrate for the first time that BDMC suppressed Adipogenesis in 3T3-L1 Adipocytes and prevented HFD-induced Obesity. Our results suggest that BDMC has the potential to Prevent Obesity.
Blumea balsamifera
Anti-Obesity Effect of Blumea balsamifera Extract in 3T3-L1 preadipocytes and Adipocytes
Obesity, the leading metabolic disease in the world, is a serious health problem in industrialized countries. We investigated the Anti-Obesity effect of Blumea balsamifera extract on Adipocyte Differentiation of 3T3-L1 preadipocytes and Anti-Obesity effect of 3T3-L1 Adipocytes. We found that treatment with an extract of Blumea balsamifera suppressed lipid accumulation and glycerol-3-phosphate dehydrogenase (GPDH) activity without affecting cell viability in 3T3-L1 preadipocytes and Adipocytes.
Furthermore, Blumea balsamifera extract brought significant attenuation of expressions of key Adipogenic transcription factors, including peroxisome proliferator-activated receptor (PPAR)γ, CCAAT element binding protein (C/EBPs) and leptin, however, induced up-regulation of adiponectin at the protein level in 3T3-L1 preadipocytes and Adipocytes. These results suggest that Blumea balsamifera extract may block Adipogenesis, at least in part, by decreasing key Adipogenic transcription factors in 3T3-L1 preadipocytes and may have antiatherogenic, anti-inflammatory, and AntiDiabetic effects through up-regulation of adiponectin in 3T3-L1 Adipocytes.
Boldine (Peumus boldus)
The Aporphine Alkaloid Boldine Induces Adiponectin Expression and Regulation in 3T3-L1 cells
Adiponectin is an adipokine secreted by differentiated Adipocytes . Clinical studies suggest a negative correlation between oxidative stress and adiponectin levels in patients with Metabolic Syndrome or cardiovascular disease. Natural compounds that can Prevent oxidative stress mediated Inhibition of adiponectin may be potentially therapeutic. Boldine, an aporphine alkaloid abundant in the medicinal plant Peumus boldus, is a powerful antioxidant. The current study demonstrates the effects of boldine on the expression of adiponectin and its regulators, CCAAT/enhancer binding protein-α (C/EBPα) and peroxisome proliferator-activated receptor (PPAR)-γ, in 3T3-L1 cells.
Differentiated 3T3-L1 Adipocytes were exposed to either hydrogen peroxide (H2O2) (100 μM) or tumor necrosis factor-α (TNFα) (1 ng/mL) for 24 hours in the presence or absence of increasing concentrations of boldine (5–100 μM). Quantitative polymerase chain reaction showed that both the oxidants decreased the mRNA levels of adiponectin, PPARγ, and C/EBPα to half of the control levels. Boldine, at all concentrations, counteracted the Inhibitory effect of H2O2 or TNFα and increased the expression of adiponectin and its regulators. The effect of boldine on adiponectin expression was biphasic, with the lower concentrations (5–25 μM) having a larger inductive effect compared to higher concentrations (50–100 μM). Boldine treatment alone in the absence of H2O2 or TNFα was also able to induce adiponectin at the inductive phase of Adipogenesis.
Peroxisome proliferator response element-luciferase promoter transactivity analysis showed that boldine interacts with the PPAR response element and could potentially modulate PPAR responsive genes. Our results indicate that boldine is able to modulate the expression of adiponectin and its regulators in 3T3-L1 cells and has the potential to be beneficial in Obesity-related cardiovascular disease.
Bromophenol (Polysiphonia morrowii)
The aim of the present study was to investigate the effect of 5‐bromo‐3,4‐dihydroxybenzaldehyde (BD) isolated from Polysiphonia morrowii on Adipogenesis and Differentiation of 3T3‐L1 preadipocytes into mature Adipocytes and its possible mechanism of action. Levels of lipid accumulation and triglyceride were significantly lower in BD treated cells than those in untreated cells. In addition, BD treatment Reduced protein expression levels of peroxisome proliferator‐activated receptor‐γ, CCAAT/enhancer‐binding proteins α, and sterol regulatory element‐binding protein 1 compared with control (no treatment).
It also Reduced expression levels of adiponectin, leptin, fatty acid synthase, and fatty acid binding protein 4. AMP‐activated protein kinase activation was found to be one specific mechanism involved in the effect of BD. These results demonstrate that BD possesses Inhibitory effect on Adipogenesis through activating AMP‐activated protein kinase signal pathway.
Butein Chalconoid
Butein is a novel Anti-Adipogenic compound
Rhus verniciflua Stokes (RVS) has been used as a traditional herbal medicine for its various biological activities including Anti-Adipogenic effects. Activity-guided separation led to the identification of the Anti-Adipogenic functions of butein. Butein, a novel Anti-Adipogenic compound, robustly suppressed lipid accumulation and Inhibited expression of Adipogenic markers. Molecular studies showed that activated transforming growth factor-β (TGF-β) and suppressed signal transducer and activator of transcription 3 (STAT3) signaling pathways were mediated by butein. Analysis of the temporal expression profiles suggests that TGF-β signaling precedes the STAT3 in the butein-mediated Anti-Adipogenic cascade.
Small interfering RNA-mediated silencing of STAT3 or SMAD2/3 blunted the Inhibitory effects of butein on Adipogenesis indicating that an interaction between two signaling pathways is required for the action of butein. Upon butein treatments, stimulation of TGF-β signaling was still preserved in STAT3 silenced cells, whereas regulation of STAT3 signaling by butein was significantly impaired in SMAD2/3 silenced cells, further showing that TGF-β acts upstream of STAT3 in the butein-mediated anti-Adipogenesis.
Capsaicin
Effects of Capsaicin on Induction of Apoptosis and Inhibition of Adipogenesis in 3T3-L1 cells
Currently, at the beginning of the 21st century, Obesity has become the leading metabolic disease in the world. It is a serious health problem in industrialized countries. Previous research has suggested that decreased Preadipocyte Differentiation and proliferation and decreased lipogenesis are mechanisms to Reduce Obesity. In the present study, the effects of capsaicin on the induction of apoptosis and Inhibition of lipid accumulation in 3T3-L1 preadipocytes and Adipocytes were investigated. The results demonstrated that capsaicin decreased cell population growth of 3T3-L1 preadipocytes, assessed with the MTT assay. Flow cytometric analysis of 3T3-L1 preadipocytes exposed to capsaicin showed that apoptotic cells increased in a time- and dose-dependent manner. Treatment with capsaicin decreased the number of normal cells and increased the number of early apoptotic and late apoptotic cells in a dose-dependent manner.
The treatment of cells with capsaicin caused the loss of mitochondria membrane potential (ΔΨm). The induction of apoptosis in 3T3-L1 preadipocytes by capsaicin was mediated through the activation of caspase-3, Bax, and Bak, and then through the cleavage of PARP and the down-regulation of Bcl-2. Moreover, capsaicin significantly decreased the amount of intracellular triglycerides and glycerol-3-phosphate dehydrogenase (GPDH) activity in 3T3-L1 Adipocytes. Capsaicin also Inhibited the expression of PPARγ, C/EBPα, and leptin, but induced up-regulation of adiponectin at the protein level. These results demonstrate that capsaicin efficiently induces apoptosis and Inhibits Adipogenesis in 3T3-L1 preadipocytes and Adipocytes.
Carduus crispus
In this study, the effects of a methanol (MeOH) extract of Carduus crispus L. (Asteraceae) on Adipogenesis was investigated in 3T3-L1 cells. To differentiate preadipocytes to Adipocytes, confluent 3T3-L1 preadipocytes were treated with a hormone mixture, which included isobutylmethylxanthine, dexamethasone, and insulin (MDI). The methanol extract of C. crispus significantly decreased Fat Accumulation by Inhibiting Adipogenic signal transcriptional factors in MDI-induced 3T3-L1 cells in a dose-dependent manner.
In MTT assays and on PI-staining, methanol extract of C. crispus Inhibited the proliferation of 3T3-L1 cells during mitotic clonal expansion (MCE). The Anti-Adipogenic effect of the Carduus extract seemed to be associated with the up-regulation of extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) pathways within the first 2 days after MDI treatment. These results suggest that methanol extract of C. crispus might be beneficial for the treatment of Obesity.
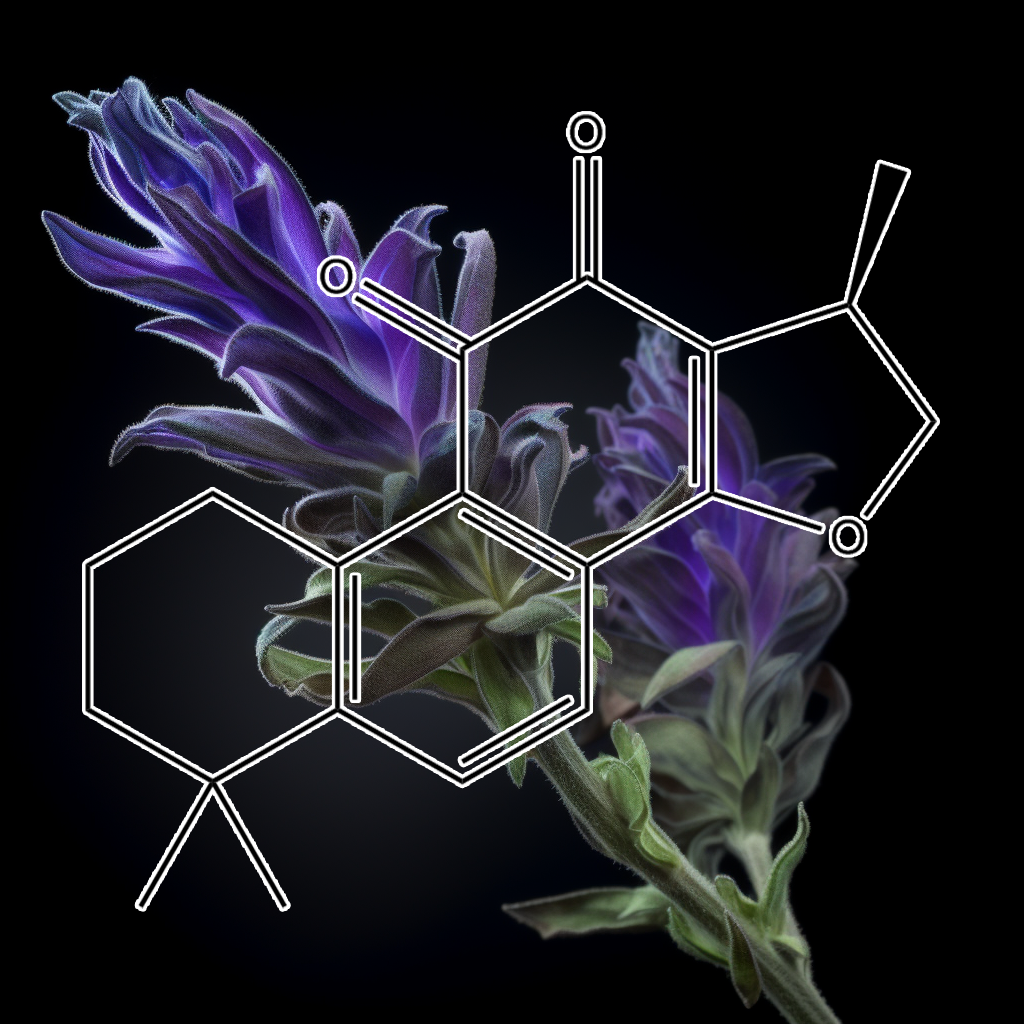
Carnosic acid (Salvia japonica Thunb.)
In the previous studies, we reported that carnosic acid (CA) and carnosol (CS) originating from rosemary protected cortical neurons by activating the Keap1/Nrf2 pathway, which activation was initiated by S-alkylation of the critical cysteine thiol of the Keap1 protein by the “electrophilic”quinone-type of CA or CS. Here, we found that CA and CS Inhibited the in vitro Differentiation of mouse preadipocytes, 3T3-L1 cells, into Adipocytes. In contrast, other physiologically-active and rosemary-originated compounds were completely negative. These actions seemed to be mediated by activation of the antioxidant-response element(ARE) and induction of phase2 enzymes. This estimation is justified by our present findings that only CA and CS among rosemary-originated compounds significantly activated the ARE and induced the phase2 enzymes. Next, we performed cDNA microarray analysis in order to identify the gene(s) responsible for these biological actions and found that phase2 enzymes (Gsta2, Gclc, Abcc4, and Abcc1), all of which are involved in the metabolism of glutathione (GSH), constituted 4 of the top 5 CA-induced genes.
Conclusions: CA exerts its Anti-Adipogenic effect in a multifactorial manner by interfering mitotic clonal expansion, altering the ratio of the different C/EBPβ forms, inducing the loss of C/EBPβ proper subnuclear distribution, and blocking the expression of C/EBPα and PPARγ.
General significance: Understanding the molecular mechanism by which CA blocks Adipogenesis is relevant because CA could be new a food additive beneficial for the prevention and/or treatment of Obesity.
Celastrol (Tripterygium wilfordii)
Objective: Celastrol, a triterpene from the root bark of the Chinese medicinal plant Tripterygium wilfordii, has been shown to exhibit anti-oxidant, anti-inflammatory, anti-cancer and insecticidal activities. Also, it has been demonstrated that celastrol has obesity-controlling effects in diet-induced obesity mice. However, direct evidence that celastrol contributes to the development of adipocyte differentiation and lipolysis has not been fully elucidated. Moreover, no previous studies have evaluated whether celastrol may regulate adipogenic transcriptional markers in adipocytes.
Materials/Methods: In order to address the questions above, we extended previous observations and investigated in vitro celastrol signaling study whether celastrol may regulate differentiation, lipolysis and key adipogenic transcriptional pathways in 3T3-L1 adipocytes.
Results: Treatment of celastrol not only inhibited adipocyte differentiation (lipid accumulation, glyceraldehyde-3-phosphate dehydrogenase activity and triglyceride content) but also increased lipolysis (glycerol release and free fatty acid release) in 3T3-L1 adipocytes. In addition, all celastrol-regulated functional activities were controlled by PPARγ2 and C/EBPα signaling pathways in duration of celastrol’s treatment in 3T3-L1 adipocytes.
Conclusion: Our initial data from in vitro celastrol signaling studies suggest novel insights into the role of PPARγ2 and C/EBPα as probable mediators of the action of celastrol in regulating adipocyte differentiation and lipolysis in 3T3-L1 adipocytes.
Chromolaena odorata
Anti-Adipogenic effect of Flavonoids from Chromolaena odorata leaves in 3T3-L1 Adipocytes
Objective: The leaves of Chromolaena odorata, a highly invasive shrub found growing wild worldwide, are traditionally used for wound healing. Due to its high flavonoidcontents, we aimed to find a new application for this plant. Preliminary tests using its ethanolic leaf extract showed that it could suppress the accumulation of lipids in Adipocytes. We therefore studied the Anti-Adipogenic effect of several C. odorata leaf extracts and the relationship between molecular structure and bio-activity of its isolated flavonoid constituents using 3T3-L1 preadipocytes /Adipocytes as a model.
Methods: Three leaf extracts and thirteen Flavonoids isolated from C. odorata were tested for their effect on lipid accumulation in 3T3-L1 Adipocytes using AdipoRed reagent, with quercetin as the positive control. The effects of active Flavonoids on the Adipocytes were confirmed by oil red O staining and visualized under a light microscope.
Results: n-Hexane and ethyl acetate extracts of C. odorata leaves displayed Anti-Adipogenic activity. The latter extract was the more potent one, especially at 40 µg/mL. Four Flavonoids, pectolinarigenin, kaempferide, 4,2′-dihydroxy-4′,5′,6′-trimethoxychalcone and dillenetin, exhibited significant, concentration-dependent Inhibitory effects on lipid accumulation in 3T3-L1 Adipocytes . The most potent flavonoid obtained in this study was 4,2′-dihydroxy-4′,5′,6′-trimethoxychalcone, which caused 75% and 90% Inhibition of cellular lipid accumulation at 30 and 50 µmol/L, respectively. Both kaempferide and 4,2′-dihydroxy-4′,5′,6′-trimethoxychalcone were major constituents in the ethyl acetate extract of this plant.
Conclusion: C. odorata leaves contained several Flavonoids with Anti-Adipogenic effects against lipid accumulation in 3T3-L1 Adipocytes. The plant, normally considered a useless weed, may actually provide an abundant source of biologically active Flavonoids.
Chrysanthemum zawadskii
Anti-Adipogenic Effects of Ethanol Extracts Prepared from Selected Medicinal Herbs in 3T3-L1 cells
Obesity is a major risk factor for various metabolic diseases such as cardiovascular disease, hypertension, and type 2 Diabetes mellitus. In this study, we prepared ethanol extracts from Agastache rugosa (ARE), Chrysanthemum zawadskii (CZE), Mentha arvensis (MAE), Perilla frutescens (PFE), Leonurus sibiricus (LSE), Gardenia jasminoides (GJE), and Lycopus coreanus (LCE). The anti-oxidant and Anti-Adipogenic effects were evaluated. The IC50 values for ascorbic acid and LCE against 2,2-diphenyl-1-picrylhydrazyl radicals were 246.2 μg/mL and 166.2 μg/mL, respectively, followed by ARE (186.6 μg/mL), CZE (198.6 μg/mL), MAE (337.1 μg/mL), PFE (415.3 μg/mL), LSE (548.2 μg/mL), and GJE (626.3 μg/mL). In non-toxic concentration ranges, CZE had a strong Inhibitory effect against 3T3-L1 adipogenes (84.5%) than those of the other extracts.
Furthermore, the Anti-Adipogenic effect of CZE is largely limited in the early stage of Adipogenesis, and we revealed that the Inhibitory role of CZE in Adipogenesis is required for the activation of Wnt signaling. Our results provide scientific evidence that the Anti-Adipogenic effect of CZE can be applied as an ingredient for the development of functional foods and nutri-cosmetics for Obesity prevention.

Chrysin (Oroxylum indicum)
Chrysin induces brown fat–like phenotype and enhances lipid metabolism in 3T3-L1 Adipocytes
Objectives: Many studies have to do with promising therapeutic phytochemicals such as Flavonoids to treat Obesity and related complications, and a number of dietary compounds have been proposed as tools for increasing energy expenditure and decreasing Fat Accumulation in mammals. Here, we show that the flavonoid chrysin induces browning of 3T3-L1 Adipocytes via enhanced expression of brown fat–specific genes and proteins as well as enhances lipid metabolism.
Methods: Chrysin-induced fat browning was investigated by determining expression levels of brown fat–specific genes and proteins by real-time polymerase chain reaction and immunoblot analysis, respectively.
Results: Chrysin enhanced expression of brown fat–specific markers and increased protein levels of peroxisome proliferator-activated receptor (PPAR)α, PPARγ, PPARδ, phosphorylated AMP-activated protein kinase (p-AMPK), phosphorylated acetyl-CoA carboxylase, hormone sensitive lipase, perilipin, carnitine palmitoyltransferase 1, acyl-coenzyme A oxidase 1, peroxisome proliferator-activated receptor-1 alpha (PGC-1α), and uncoupling protein 1 (UCP-1), suggesting its possible role in augmentation of Lipolysis, fat oxidation, and thermogenesis as well as reduction of lipogenesis. Increased expression of UCP-1 and other brown fat–specific markers was possibly mediated by chrysin-induced activation of AMPK based on the fact that Inhibition of AMPK by dorsomorphin abolished expression of PR domain-containing 16, UCP-1, and PGC-1α while the activator 5-aminoimidazole-4-carboxamide ribonucleotide elevated expression of these brown marker proteins.
Conclusion: Our findings suggest that chrysin plays a dual modulatory role in the form of inducing the brown-like phenotype as well as enhancing lipid metabolism and thus may be explored as a potentially promising food additive for prevention of Obesity.
Cinnamomum verum
This study was performed to evaluate the AntiObesity effect of supercritical fluid extracts (SFC) and marc methanol extracts (SFM) from Cinnamomum verum in 3T3-L1 preadipocytes. In inducing the Differentiation of 3T3-L1 preadipocytes in the presence of an Adipogenic cocktail, iso-butylmethylanthine (IBMX), dexamathasone, and insulin, treatment with fraction residue SFC and SFM. SFC significantly Reduced the mRNA expression of the transcription factor peroxisome proliferator-activatedreceptor-γ (PPARγ), the sterol regulatory-element-binding protein-1c (SREBP1c), and the CCAAT enhancer-binding-protein α (C/EBPα) in a concentration-dependent manner.
Cirsium brevicaule
Cirsium brevicaule A. GRAY leaf Inhibits Adipogenesis in 3T3-L1 cells and C57BL/6 mice
Results: Treatment of 3T3-L1 Adipocytes with a hexane extract of CL significantly Reduced cellular lipid accumulation and expression of the fatty acid synthase (FASN) gene. Dietary CL Reduced the serum levels of non-esterified fatty acids in HFD-fed mice. Significant decreases in subcutaneous WAT weight and associated FASN gene expression were observed in the mice fed the experimental CL diet. Dietary CL also Reduced the hepatic lipid and serum levels of a hepatopathic indicator in the HFD-fed mice. A significant reduction in mRNA levels of FASN and HMG-CoA reductase were observed in the livers of the CL-diet group. Dietary CL, on the other hand, increased in the hepatic mRNA levels of genes related to β-oxidation, namely peroxisome proliferator-activated receptor α, calnitine palmitoyltrasferase 1A, and uncoupling protein 2. Expression of the insulin receptor gene was also significantly increased in the livers of mice-fed the CL diet.
Conclusions: The present study therefore demonstrated that CL Suppresses lipid accumulation in the WAT and liver partly through Inhibiting mRNA levels of FASN gene and enhancing the Lipolysis -related gene expression.
Cirsium setidens
Cirsium setidens Nakai, a wild perennial herb, grows mainly in Gangwon province, Korea, and has been reported to contain bioactive ingredients with various medicinal activities, including the treatment of edema, bleeding, and hemoptysis. However, the potential AntiObesity effects of C. setidens Nakai have not been fully investigated. This study evaluated the AntiObesity effect of standardized C. setidens Nakai ethanolic extract (CNE) in 3T3-L1 Adipocytes and in obese C57BL/6J mice fed a high-fat diet. CNE suppressed the expression of lipogenic genes and increased the expression of lipolytic genes. The AntiAdipogenic and antilipogenic effects of CNE appear to be mediated by the Inhibition of peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer-binding protein (C/EBP) expressions.
Moreover, CNE stimulated fatty acid oxidation in an AMPK-dependent manner. CNE-treated groups of C57BL/6J mice showed Reduced body weights and Adipose Tissue weight and improved serum lipid profiles through the downregulation of PPARγ, C/EBPα, fatty acid binding protein 4 (FABP4), sterol regulatory element binding protein-1c (SREBP-1c), and fatty acid synthase (FAS) and the upregulation of adiponectin and carnitine palmitoyltransferase-1 (CPT-1) in obese C57BL/6J mice fed a high-fat diet. These results suggest that CNE may have an AntiObesity effect on Adipogenesis and lipid metabolism in vitro and in vivo and present the possibility of developing a treatment for Obesity with nontoxic natural resources.
Cirsium setidens Nakai contains bioactive compounds that exert biological activities. Method validation for analysis of the pectolinarin content in Cirsium setidens extracts (CSE) and radical scavenging-linked AntiObesity activities using 3T3-L1 cells and C57BL/6 mice were performed. The pectolinarin content of CSE was 2.81±0.01 mg/g with a high degree of linearity in calibration curves (R2=0.9999).
CSE exhibited free radical-scavenging activities and a reducing power. CSE and pectolinarin Inhibited lipid accumulation during Adipogenesis of 3T3-L1 cells via down-regulation of Adipogenic transcription factors. CSE supplementation suppressed body weight in C57BL/6 mice fed a high fat diet and Reduced plasma total cholesterol, triglyceride, insulin, and glucose levels. Pectolinarin-enriched CSE can be considered as a good source of natural Antioxidants and AntiObesity ingredients.
Citrus aurantium
Citrus aurantium Flavonoids Inhibit Adipogenesis through the Akt signaling pathway in 3T3-L1 cells
Background: Obesity is a health hazard that is associated with a number of diseases and metabolic abnormalities, such as type-2 Diabetes , hypertension, dyslipidemia, and coronary heart disease. In the current study, we investigated the effects of Citrus aurantium Flavonoids (CAF) on the Inhibition of Adipogenesis and Adipocyte Differentiation in 3T3-L1 cells.
Methods: During Adipocyte Differentiation, 3T3-L1 cells were treated with 0, 10, and 50 μg/ml CAF, and then the mRNA and protein expression of Adipogenesis-related genes was assayed. We examined the effect of CAF on level of phosphorylated Akt in 3T3-L1 cells treated with CAF at various concentrations during Adipocyte Differentiation.
Results: The insulin-induced expression of C/EBPβ and PPARγ mRNA and protein were significantly down-regulated in a dose-dependent manner following CAF treatment. CAF also dramatically decreased the expression of C/EBPα, which is essential for the acquisition of insulin sensitivity by Adipocytes. Moreover, the expression of the aP2 and FAS genes, which are involved in lipid metabolism, decreased dramatically upon treatment with CAF. Interestingly, CAF diminished the insulin-stimulated serine phosphorylation of Akt (Ser473) and GSK3β (Ser9), which may Reduce glucose uptake in response to insulin and lipid accumulation. Furthermore, CAF not only Inhibited triglyceride accumulation during Adipogenesis but also contributed to the Lipolysis of Adipocytes.
Conclusions: In the present study, we demonstrate that CAF suppressed Adipogenesis in 3T3-L1 Adipocytes . Our results indicated that CAF down-regulates the expression of C/EBPβ and subsequently Inhibits the activation of PPARγ and C/EBPα. The Anti-Adipogenic activity of CAF was mediated by the Inhibition of Akt activation and GSK3β phosphorylation, which induced the down-regulation of lipid accumulation and lipid metabolizing genes, ultimately Inhibiting Adipocyte Differentiation.
Clitoria ternatea
Clitoria ternatea (commonly known as blue pea) flower petal extract (CTE) is used as a natural colorant in a variety of foods and beverages. The objective of study was to determine the Inhibitory effect of CTE on Adipogenesis in 3T3-L1 preadipocytes. The phytochemical profiles of CTE were analyzed by liquid chromatography and tandem Mass spectrometry (LC-MS/MS). Anti-Adipogenesis effect of CTE was measured by using Oil Red O staining, intracellular triglyceride assay, quantitative real-time PCR and western blot analysis in 3T3-L1 Adipocytes. Cell cycle studies were performed by flow cytometry. Lipolysis experiments were performed using a colorimetric assay kit. In early stages, CTE demonstrated Anti-Adipogenic effects through Inhibition of proliferation and cell cycle retardation by suppressing expression of phospho-Akt and phospho-ERK1/2 signaling pathway.
The results also showed that CTE Inhibited the late stage of Differentiation through diminishing expression of Adipogenic transcription factors including PPARγ and C/EBPα. The Inhibitory action was subsequently attenuated in downregulation of fatty acid synthase and acetyl-CoA carboxylase, causing the reduction of TG accumulation. In addition, CTE also enhanced catecholamine-induced Lipolysis in Adipocytes. These results suggest that CTE effectively attenuates Adipogenesis by controlling cell cycle progression and downregulating Adipogenic gene expression.
Camellia ptilophylla
Cocoa tea (Camellia ptilophylla) is a naturally decaffeinated tea plant. Previously we found that cocoa tea demonstrated a beneficial effect against high-fat diet induced Obesity , hepatic steatosis and hyperlipidemia in mice. The present study aimed to investigate the Anti-Adipogenic effect of cocoa tea in vitro using preadipocytes 3T3-L1. Adipogenic Differentiation was confirmed by Oil Red O stain, qPCR and Western blot. Our results demonstrated that cocoa tea significantly Inhibited triglyceride accumulation in mature Adipocytes in a dose-dependent manner. Cocoa tea was shown to suppress the expressions of key Adipogenic transcription factors, including peroxisome proliferator-activated receptor gamma (PPAR γ) and CCAAT/enhancer binding protein (C/EBP α).
Colocynth (Citrullus colocynthis)
Adipogenesis is the overall process of Adipocyte Differentiation. With a positive energy balance, intracellular accumulation of triglyceride increases through the generation of functional Adipocytes. This occurs with Adipogenesis of undifferentiated preadipocytes. Citrullus colocynthis (CC), a member of the Cucurbitaceae family, is a desert viny plant native to North Africa. The effect of ethanolic seed extracts of colocynth (SCEE) on Adipogenesis was investigated using 3T3-L1 preadipocytes. Cell viability was measured using the MTT assay; triglycerides were stained with Oil Red O and Adipogenesis-related gene expressions were quantified using qRT-PCR. Results showed that SCEE helps to Inhibit intracellular triglyceride accumulation during Adipogenesis without affecting cell viability. Likewise, SCEE not only showed Anti-Adipogenic activities, essentially during the early stage, but their effects during the middle and late stages were very low.
Coptis chinensis
Objectives: This study was to investigate the antioxiative capacity, AntiObesity effects of Atractylodes Rhizoma Alba, Houttuyniae Herba, Lonicerae Flos, Scutellariae Radix, and Coptidis Rhizoma on Raw 264.7 and 3T3-L1 cell lines.
Methods: Three different types of herb extracts (A. Rhizoma Alba, H. Herba, L. Flos, S. Radix, and C. Rhizoma; water 100%, ethanol 30%, ethanol 100%) were used in this study. Total Polyphenol compound, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging, reactive oxygen species (ROS) activity, NO production and cell proliferation were measured.
Results: Total Polyphenol compound measurement of L. Flos, A. Rhizogenes, and C. Rhizoma extracts were higher than A. Rhizoma Alba, H. Herba. DPPH radical scavenging activity, ROS activity and NO production of S. Radix, C. Rhizoma extracts were lower than L. Flos, A. Rhizoma, and H. Herba.
Conclusions: Metformin and S. Radix, C. Rhizoma, A. Rhizoma Alba, and L. Flos extracts combination groups showed synergistic effect on Adipocyte Differentiation Inhibition and antioxidative activity.
Cordycepin (Cordyceps sinensis)
This study aimed to investigate the effects of Cordyceps sinensis extract (CSE) and Gymnema inodorum extract (GIE), used alone and combined, on AntiAdipogenesis in 3T3-L1 cells . Oil Red O staining was used to examine the effects of these extracts on Inhibition of intracellular lipid accumulation in 3T3-L1 Adipocytes and on lipid droplet morphology. Fourier transform-infrared (FTIR) microspectroscopy was used to examine biomolecular changes in 3T3-L1 Adipocytes . The pancreatic lipase assay was used to evaluate the Inhibitory effects of CSE and GIE on pancreatic lipase activity.
Taken together, the results indicated that CSE, GIE, and their combination suppressed lipid accumulation. The FTIR microspectroscopy results indicated that CSE, GIE, and their combination had Inhibitory effects on lipid accumulation in the Adipocytes. Compared with the untreated Adipocytes, the signal intensity and integrated areas of glycogen and other carbohydrates, the acyl chain of phospholipids, and the lipid/protein ratios of the CSE, GIE, alone, and combined treated Adipocytes were significantly lower (p < 0.05). Combination treatment resulted in a synergistic effect on lipid accumulation reduction in the Adipocytes. Principal component analysis of the biomolecular changes revealed six distinct clusters in the FTIR spectra of the sample cells. The pancreatic lipase assay results indicated that CSE and GIE Inhibited the pancreatic lipase activity in a dose-dependent manner (mean ± standard error of the mean IC50 values, 2312.44 ± 176.55 μg mL−1 and 982.24 ± 44.40 μg mL−1, resp.). Our findings indicated that FTIR microspectroscopy has potential application for evaluation of the effectiveness of medicinal plants and for the development of infrared biochemical Obesity markers useful for treating patients with Obesity.
Coumestrol
Coumestrol modulates Akt and Wnt/β-catenin signaling during the attenuation of Adipogenesis
Coumestrol is a natural phytochemical present in plants such as red clover and soy, and has been reported to stimulate the estrogen receptor as a major phytoestrogen. While the molecular mechanisms responsible for the Anti-Adipogenic effects of phytoestrogens such as genistein and daidzein have been previously investigated, the effects of coumestrol on Adipogenesis remain to be elucidated. We observed that coumestrol dose-dependently attenuates MDI (mixture of 3-isobutyl-1-methylxanthine, dexamethasone, and insulin)-induced lipid accumulation, consistent with an earlier study, while significantly Inhibiting MDI-induced Adipogenesis in the first 48 hours of Differentiation, a critical time window for Anti-Adipogenic effects. Coumestrol treatment suppressed MDI-induced protein expression of PPARγ and C/EBPα in Adipocytes, leading to the subsequent downregulation of FAS and aP2 expression. Akt and GSK3β were phosphorylated shortly after MDI stimulation, and these responses were Inhibited by coumestrol treatment.
Coumestrol also increased LRP6 protein expression, resulting in the recovery of β-catenin downregulation by MDI, while attenuating MDI-induced downregulation of Wnt10b. In addition, mRNA and protein expression of c-Myc and cyclin D1, target genes of β-catenin, were both recovered by coumestrol treatment. These results suggest that coumestrol Inhibits Adipocyte Differentiation via regulation of Akt and Wnt/β-catenin signaling and may have potential for development as an agent to Prevent Adipogenesis.
Cranberry (Oxycoccus quadripetalus)
Cranberries (Oxycoccus quadripetalus) Inhibit Adipogenesis and lipogenesis in 3T3-L1 cells
Cryptotanshinone (Salvia miltiorrhiza)
Background: Cryptotanshinone (CT), a major tanshinonefound in Salvia miltiorrhiza Bunge (Lamiaceae), has various pharmacological effects such as antitumor, anti-inflammatory, and antioxidant properties. Despite its well-documented benefits in a wide range of diseases, the effect of CT on Adipocyte Differentiation has not been well characterized.
Purpose: The present study was designed to determine the in vitro Anti-Adipogenic effect and underlying molecular mechanisms of CT using 3T3-L1 murine Pre-Adipocytes .
Methods: We measured the levels of intracellular triglyceride accumulation and mRNA and protein expression of key Adipogenic transcription factors and their target genes.
Results: Treatment with CT drastically Reduced lipid accumulation in a dose- and time-dependent manner. Molecular assays showed that CT effectively suppressed the expression of C/EBPβ, C/EBPα , and PPARγ and of their target Adipocyte -specific genes aP2, adiponectin, and GLUT4 but activated the expression of Anti-Adipogenic genes such as GATA2, CHOP10, and TNF-α. CT treatment also Inhibited the phosphorylation of STAT3 in the early phase of Adipogenesis . A small-interfering-RNA-mediated knock-down of STAT3 potentiated the Anti-Adipogenic effect of CT.
Conclusion: Taken together, the results suggest that CT may be a good Anti-Adipogenic candidate because it regulates STAT3 during early Adipogenesis.
Curcumin
Conjugation of curcumin (CCM) by polyethylene glycol (PEG) has been previously developed to improve water solubility of the natural form of CCM and its antiproliferative role in some human cancer cell lines. This study examined the cellular uptake kinetics of the natural form of CCM and CCM−PEG. Their cytotoxic effect in proliferating preadipocytes and AntiAdipogenic property in differentiating preadipocytes had also been investigated. CCM and CCM−PEG were found to be differently absorbed in 3T3-L1 preadipocytes and Adipocytes with a limited amount of CCM−PEG absorption in the cell. The improved water solubility of CCM−PEG was correlated with increased cellular retention of CCM in 3T3-L1 cells, particularly in preadipocytes.
Consequently, CCM−PEG treatment sensitized proliferating preadipocytes to CCM-induced cell toxicity. Furthermore, incubation of differentiating 3T3-L1 cells with CCM−PEG resulted in improvement of the Inhibitory role of CCM in Adipocyte Differentiation with no toxic effect. These results suggest that pegylation-improved water solubility and cellular retention of CCM may be uniquely useful for improving the delivery of CCM in preadipocytes and its AntiAdipogenic ability.
Curcumin Inhibits Adipogenesis in 3T3-L1 Adipocytes and angiogenesis and Obesity in C57/BL mice
Angiogenesis is necessary for the growth of Adipose Tissue. Dietary Polyphenols may suppress growth of Adipose Tissue through their antiangiogenic activity and by modulating Adipocyte metabolism. We investigated the effect of curcumin, the major Polyphenol in turmeric spice, on angiogenesis, Adipogenesis, Differentiation, apoptosis, and gene expression involved in lipid and energy metabolism in 3T3-L1 Adipocyte in cell culture systems and on body Weight Gain and Adiposity in mice fed a high-fat diet (22%) supplemented with 500 mg curcumin/kg diet for 12 wk. Curcumin (5–20 μmol/L) suppressed 3T3-L1 Differentiation, caused apoptosis, and Inhibited adipokine-induced angiogenesis of human umbilical vein endothelial cells.
Supplementing the high-fat diet of mice with curcumin did not affect food intake but Reduced body Weight Gain, Adiposity, and microvessel density in Adipose Tissue, which coincided with Reduced expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2. Curcumin increased 5′AMP-activated protein kinase phosphorylation, Reduced glycerol-3-phosphate acyl transferase-1, and increased carnitine palmitoyltransferase-1 expression, which led to increased oxidation and decreased fatty acid esterification. The in vivo effect of curcumin on the expression of these enzymes was also confirmed by real-time RT-PCR in subcutaneous Adipose Tissue. In addition, curcumin significantly lowered serum cholesterol and expression of PPARγ and CCAAT/enhancer binding protein α, 2 key transcription factors in Adipogenesis and lipogenesis. The curcumin suppression of angiogenesis in Adipose Tissue together with its effect on lipid metabolism in Adipocytes may contribute to lower body fat and body Weight Gain. Our findings suggest that dietary curcumin may have a potential benefit in preventing Obesity.
Cyanidine-3-O-Galactoside (Aronia melanocarpa)
Aronia melanocarpa are a rich source of Anthocyanins that have received considerable interest for their relations to human health. In this study, the Anti-Adipogenic effect of cyanidin-3-O-galactoside-enriched Aronia melanocarpa extract (AM-Ex) and its underlying mechanisms were investigated in an in vivo system. Five-week-old male C57BL/6N mice were randomly divided into five groups for 8-week feeding with a control diet (CD), a high-fat diet (HFD), or a HFD with 50 (AM-Ex 50), 100 (AM-Ex 100), or 200 AM-Ex (AM-Ex 200) mg/kg body weight/day. HFD-fed mice showed a significant increase in body weight compared to the CD group, and AM-Ex dose-dependently Inhibited this Weight Gain. AM-Ex significantly Reduced the food intake and the weight of white fat tissue, including epididymal fat, retroperitoneal fat, mesenteric fat, and inguinal fat. Treatment with AM-Ex (50 to 200 mg/kg) Reduced serum levels of leptin, insulin, triglyceride, total cholesterol, and low density lipoprotein (LDL)-cholesterol.
Real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis revealed that AM-Ex suppressed Adipogenesis by decreasing CCAAT/enhancer binding protein α, peroxisome proliferator-activated receptor γ, sterol regulatory element-binding protein-1c, peroxisome proliferator-activated receptor gamma coactivator-1α, acetyl-CoA carboxylase 1, ATP-citrate lyase, fatty acid synthase, and Adipocyte protein 2 messenger RNA (mRNA) expressions. These results suggest that AM-Ex is potentially beneficial for the suppression of HFD-induced Obesity by modulating multiple pathways associated with Adipogenesis and food intake.
Our results showed that the Anti-Obesity effect of AM-Ex occured through down-regulation of the transcription factors PPARγ, C/EBPα, SREBP-1c, and Adipogenesis and lipogenesis-related genes, aP2, LPL, ACC1, ACL, and FAS. These results demonstrate that AM-Ex can be used as a preventive or therapeutic agent for Obesity. Future, animal and human studies are needed to further investigate the mechanism and proper concentration of AM to be used as Anti-Obesity agents.
Cyclopia subternata
The stems, leaves and flowers of Cyclopia have been consumed as aherbal tea ‘honeybush tea’ to treat various medical ailments since the 19th century. Plant Polyphenols are reported to Inhibit Adipogenesis in cell and animal models of Obesity. The aim of this study was to assess the effect of hot water extracts of two Cyclopia species, C. maculata and C. subternata on Obesity in an in vitro model. The total Polyphenol content of unfermented C. subternata, unfermented C. maculata and fermented C. maculata extracts was 25.6, 22.4 and 10.8 g GAE/100 g, respectively.
The major compounds present in the extracts were: the flavonoid, phloretin-3′,5′-di-C-glucoside in C. subternata, the xanthone, mangiferin in unfermented C. maculata and the flavanone, hesperidin in fermented C. maculata. All of the plant extracts Inhibited intracellular triglyceride and Fat Accumulation, and decreased PPARγ 2 expression. The higher concentrations of unfermented C. maculata (800 and 1600 μg/ml) and C. subternata(1600 μg/ml) were cytotoxic in terms of decreased mitochondrialdehydrogenase activity. Both fermented and unfermented C. maculata, at concentrations greater than 100 μg/ml, decreased cellular ATPcontent. Cyclopia maculata and C. subternata Inhibit Adipogenesis in vitro, suggesting their potential as Anti-Obesity agents.
Cyclopia maculata (honeybush tea) stimulates Lipolysis in 3T3-L1 Adipocytes
We have previously, for the first time, demonstrated that hot water extracts of Cyclopia maculata and Cyclopia subternata, endemic South African plants that are consumed as herbal teas, Inhibit Adipogenesis in 3T3-L1 Adipocytes. The aim of this study was to extend the Anti-Obesity investigations of these plants by quantifying Lipolysis in mature 3T3-L1 Adipocytes. Glycerol concentration in culturesupernatants was used as a marker of Adipocyte Lipolysis. Isoproterenol, a β-adrenergic agonist and a known lipolytic agent, was used as a positive control in our assays. Lipolysis was stimulated by all extracts, although statistical significance was noted for fermented (oxidised) C. maculata only. A concentration of 80 μg/ml ofC. maculata extract induced maximal Lipolysis (1.8-fold, p < 0.001).
The increased Lipolysis was accompanied by an increase in the expression of hormone sensitive lipase (1.6-fold, p < 0.05) and perilipin (1.6-fold,p < 0.05). The plant extracts, at the concentration range assayed (0–100 μg/ml), were not cytotoxic in terms of mitochondrialdehydrogenase and adenosine-5′-triphosphate activity. These results showed that C. maculata stimulates Lipolysis in mature 3T3-L1 Adipocytes, providing further support for the Anti-Obesity effects ofCyclopia spp.
Delphinidin-3-O–β-glucoside (D3G) is a health-promoting anthocyanin whose Anti-Obesity activity has not yet been thoroughly investigated. We examined the effects of D3G on Adipogenesis and lipogenesis in 3T3-L1 Adipocytes and primary white Adipocytes using real-time RT-PCR and immunoblot analysis. D3G significantly Inhibited the accumulation of lipids in a dose-dependent manner without displaying cytotoxicity. In the 3T3-L1 Adipocytes, D3G downregulated the expression of key Adipogenic and lipogenic markers, which are known as peroxisome proliferator-activated receptor gamma (PPARγ), sterol regulatory element-binding transcription factor 1 (SREBP1), CCAAT/enhancer-binding protein alpha (C/EBPα ), and fatty acid synthase (FAS).
Moreover, the relative protein expression of silent mating type information regulation 2 homolog 1 (SIRT1) and carnitine palmitoyltransferase-1 (CPT-1) were increased, alongside Reduced lipid levels and the presence of several small lipid droplets. Furthermore, D3G increased the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) and acetyl-CoA carboxylase (ACC), which suggests that D3G may play a role in AMPK and ACC activation in Adipocytes. Our data indicate that D3G attenuates Adipogenesis and promotes lipid metabolism by activating AMPK-mediated signaling, and, hence, could have a therapeutic role in the management and treatment of Obesity.
Abstract Delphinidin–3–O–β–glucoside (D3G) is a health-promoting anthocyanin whose anti–Obesity activity has not yet been thoroughly investigated. We examined the effects of D3G on Adipogenesis and lipogenesis in 3T3-L1 Adipocytes and primary white Adipocytes.
DHEA
Dehydroepiandrosterone (DHEA), a precursor sex steroid, circulates in sulphated form (DHEAS). Serum DHEAS concentrations are inversely correlated with Metabolic Syndrome components and in vivo/in vitro studies suggest a role in modulating adipose Mass. To investigate further, we assessed the in vitro biological effect of DHEA in white (3T3-L1 ) and brown (PAZ6) Preadipocyte cell lines and human primary preadipocytes.
DHEA (from 10−8 M) caused concentration-dependent proliferation Inhibition of 3T3-L1 and PAZ6 preadipocytes. Cell cycle analysis demonstrated unaltered apoptosis but indicated blockade at G1/S or G2/M in 3T3-L1 and PAZ6, respectively. Preadipocyte cell-line Adipogenesis was not affected.
In human primary subcutaneous and omental preadipocytes, DHEA significantly Inhibited proliferation from 10−8 M. DHEA 10−7 M had opposing effects on Adipogenesis in the two fat depots. Subcutaneous Preadipocyte Differentiation was unaffected or increased whereas omental preadipocytes showed significantly Reduced Adipogenesis.
We conclude that DHEA exerts fat depot-specific differences which modulate body composition by limiting omental fat production.
Diallyl trisulphide (onion)
The aim of the present study was to examine the effect of quercetin-rich onion peel extract (OPE) on anti-Differentiation in 3T3-L1 preadipocytes and the AntiObesity in high-fat fed rats. We found that lipid accumulations and TG contents in 3T3-L1 cells were markedly suppressed by OPE. The mRNA levels of activating protein (AP2) were down-regulated and those of carnitine palmitoyl transferase-1 α (CPT-1α) and fatty acid binding protein 4 (FABP4) were up-regulated by 75 and 100 μg/ml OPE. Body weight, retroperitoneal and mesenteric fat weights of SD rats were significantly lower in the 8 week high fat (HF) diet + 0.72% OPE group than in the HF group.
Peroxisome proliferator-activated receptor (PPAR)γ mRNA levels were down-regulated in the epididymal fat of OPE than those of control and HF, and significant down-regulation of CCAAT/enhancer binding protein (C/EBP)α mRNA levels in OPE was also observed than the control. The mRNA levels of CPT-1α and uncoupling protein-1 (UCP-1) were up-regulated by the OPE, while those of fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) were down-regulated in HF and OPE groups compared to control group. These results suggest that quercentin-enriched OPE may have AntiObesity effects by suppressing Preadipocyte Differentiation and Inhibiting Adipogenesis.
Dioscin (Dioscorea japonica)
Dioscin (DS) is a steroidal saponin present in a number of medicinal plants and has been shown to exert anticancer, antifungal and antiviral effects. The present study aimed to deternube the effects DS on the regulation of Adipogenesis and to elucidate the underlying mechanisms. In vitro experiments were performed using differentiating 3T3-L1 cells treated with various concentrations (0-4 µM) of DS for 6 days. A cell viability assay was performed on differentiating cells following exposure to DS. Oil Red O staining and triglyceride content assay were performed to evaluate the lipid accumulation in the cells. We also carried out the following experiments: i) flow cytometry for cell cycle analysis, ii) quantitative reverse transcription polymerase chain reaction for measuring Adipogenesis-related gene expression, and iii) western blot analysis to measure the expression of Adipogenesis transcription factors and AMP-activated protein kinase (AMPK), acetyl-CoA carboxylase (ACC) and mitogen-activated protein kinase (MAPK) phosphorylation.
In vivo experiements were performed using mice with Obesity induced by a high-fat diet (HFD) that were treated with or without DS for 7 weeks. DS suppressed lipid accumulation in the 3T3-L1 cells without affecting viability at a dose of up to 4 µM. It also delayed cell cycle progression 48 h after the initiation of Adipogenesis. DS Inhibited Adipocyte Differentiation by the downregulation of Adipogenic transcription factors and attenuated the expression of Adipogenesis-associated genes. In addition, it enhanced the phosphorylation of AMPK and its target molecule, ACC, during the Differentiation of the cells. Moreover, the Inhibition of Adipogenesis by DS was mediated through the suppression of the phosphorylation of MAPKs, such as extracellular-regulated kinase 1/2 (ERK1/2) and p38, but not c-Jun-N-terminal kinase (JNK).
DS significantly Reduced Weight Gain in the mice with HFD-induced Obesity; this was evident by the suppression of Fat Accumulation in the abdomen. the present study reveals an Anti-Adipogenic effect of DS in vitro and in vivo and highlights AMPK/MAPK signaling as targets for DS during Adipogenesis.
Chenopodium formosanum
The aim of this study was to provide new insights into the role of the ethanolic extracts of Djulis (Chenopodium formosanum, EECF) and its bioactive compounds in preventing Adipogenesis in 3T3-L1 Adipocytes. The results demonstrated EECF significantly Inhibited oil red O-stained material (OROSM), triglyceride levels and glycerol-3-phosphate dehydrogenase (GPDH) activity in 3T3-L1 Adipocytes. The expression of the critical molecules involved in lipid synthesis such as PPARγ, C/EBPα and SREBP-1c was attenuated in EECF-treated cells. According to HPLC-DAD and HPLC-MS/MS analysis, rutin, kaempferol, betanin and another nine compounds were present in EECF. The suppression of lipid accumulation by rutin, kaempferol and betanin occurred by decreasing the gene expression of PPARγ, C/EBPα and SREBP-1c. Taken together, these findings suggest the presence of bioactive compounds in EECF may partly account for the anti-Adipogenesis of EECF and EECF is therefore a potentially lipid lowering functional food.
Dolichos lablab
Anti–Obesity activities of chikusetsusaponin IVa and Dolichos lablab L. seeds
Obesity, a condition where excess body fat accumulates to the extent, causes a negative effect on health. Previously, we reported the extract of Dolichos lablab L. (DLL-Ex) Inhibited high-fat diet (HFD)-induced increases in body weight and body fat Mass and ameliorated increases in body weight. In the present work, we studyed the molecular mechanism for the Inhibitory effect of DLL-Ex or Chikusetsusaponin IVa (CS-IVa), as isolated from Dolichos lablab L. (DLL) seeds extract, on Adipocyte Differentiation . We evaluated the effect of DLL-Ex, an Anti-Obesity agent, and CS-IVa, an active component of DLL-Ex, on 3T3-L1 cell Differentiation via Oil red O assay and Q-PCR, along with their effects on CCAAT element binding protein alpha (C/EBPα ), peroxisome proliferator-activated receptor gamma (PPARγ), fatty acid synthase (FAS), and fatty acid-binding protein 4 (FABP4) mRNA transcriptions. FAS and FABP4 protein expression levels after exposure to CS-IVa were also tested. The results showed that DLL-Ex and CS-IVa have potent Inhibitory activity on Adipocyte Differentiation. Therefore, DLL and CS-IVa may be developed as a functional food material to treat Obesity.

Ecklonia Cava
•The Ecklonia cava extract tested herein evidenced profound Adipogenesis Inhibition.
•The three Polyphenol compounds of phlorotannins were isolated from E. cava.
•Dieckol exhibited greatest potential Adipogenesis Inhibition.
•Dieckol Inhibits Adipogenesis by activating the AMPK pathway.
In this study, we assessed the potential Inhibitory effect of 5 species of brown seaweeds on Adipogenesis the Differentiation of 3T3-L1 preadipocytes into mature Adipocytes by measuring Oil-Red O staining. The Ecklonia cava extract tested herein evidenced profound Adipogenesis Inhibitory effect, compared to that exhibited by the other four brown seaweed extracts. Thus, E. cava was selected for isolation of active compounds and finally the three Polyphenol compounds of phlorotannins were obtained and their Inhibitory effect on Adipogenesis was observed. Among the phlorotannins, dieckol exhibited greatest potential Adipogenesis Inhibition and down-regulated the expression of peroxisome proliferator-activated receptor-γ (PPARγ), CCAAT/enhancer-binding proteins (C/EBPα), sterol regulatory element-binding protein 1 (SREBP1) and fatty acid binding protein 4 (FABP4) in a dose-dependent manner.
The specific mechanism mediating the effects of dieckol was confirmed by AMP-activated protein kinase (AMPK) activation. These results demonstrate Inhibitory effect of dieckol compound on Adipogenesis through the activation of the AMPK signal pathway.
Anti-Adipogenic effect of dioxinodehydroeckol via AMPK activation in 3T3-L1 Adipocytes
In this study, we examined the Inhibitory effects of enzyme- treated Ecklonia cava (EEc) extract on the Adipogenesis of 3T3-L1 Adipocytes. The components of Ecklonia cava (E. cava) were first separated and purified using the digestive enzymes pectinase (Rapidase® X‑Press L) and cellulase (Rohament® CL). We found that the EEc extract contained three distinct phlorotannins: eckol, dieckol and phlorofucofuroeckol-A. Among the phlorotannins, dieckol was the most abundant in the EEc extract at 16 mg/g. Then we examined the Inhibitory effects of EEc extract treatment on Differentiation‑related transcription factors and on Adipogenesis‑related gene expression in vitro using 3T3-L1 Adipocytes. 3T3‑L1 pre‑Adipocytes were used to determine the concentrations of the EEc extract and Garcinia cambogia (Gar) extract that did not result in cytotoxicity. Glucose utilization and triglyceride (TG) accumulation in the EEc‑treated Adipocytes were similarly Inhibited by 50 µg/ml EEc and 200 µg/ml Gar, and these results were confirmed by Oil Red O staining.
Protein expression of Adipogenesis Differentiation‑related transcription factors following treatment with the EEc extract was also examined. Only the expression of CCAAT/enhancer‑binding protein (C/EBP)α was decreased, while there was no effect on the expression of C/EBPβ, C/EBPδ, and peroxisome proliferator‑activated receptor γ (PPARγ). Treatment with the EEc extract decreased the expression levels of Adipogenesis‑related genes, in particular sterol regulatory element binding protein‑1c (SREBP‑1c), Adipocyte fatty acid binding protein (A‑FABP), fatty acid synthase (FAS) and adiponectin. These results suggest that EEc extract treatment has an Inhibitory effect on Adipogenesis, specifically by affecting the activation of the C/EBPα signaling pathway and the resulting Adipogenesis-related gene expression.
Ecliptal (Eclipta alba)
A swift increase has been observed in the number of individuals with metabolic syndrome worldwide. A number of natural compounds have been identified towards combating metabolic syndrome. Adding to this premise, here we report the pleiotropic activities of Ecliptal (EC); a natural compound isolated from the herb Eclipta alba. Administration of EC was shown to have prominent anti-adipogenic effects in 3T3-L1 and hMSC derived adipocytes. It was shown to activate Wnt-pathway and alter AKT signaling. Additionally, it caused cell cycle arrest and inhibited mitotic clonal expansion. EC treatment augmented mitochondrial biogenesis as well as function as estimated by expression of PGC1α, UCP-1, mitochondrial complexes and estimation of oxygen consumption rate. EC also reduced LPS-induced inflammation and tunicamycin induced ER stress.
Further, EC enhanced insulin sensitivity by increasing AKT phosphorylation, inhibiting PKCα/βII phosphorylation and reducing leptin/adiponectin ratio. Finally, EC administration in Syrian golden hamsters was shown to have potent anti-dyslipidemic effects. Cumulatively, encompassing pleiotropic activities of EC, it could prove to be a potential drug candidate against obesity, insulin resistance and related metabolic syndrome.
Ethyl acetate fraction of Eclipta alba: a potential phytopharmaceutical targeting Adipocyte Differentiation
Egcg
(-)Epigallocatechin gallate (EGCG) is the most abundant catechin in green tea and reportedly has anti-obesity and anti-adipogenic effects. In this study, we determined that the up-regulation of the WNT/β-catenin pathway is the anti-adipogenic mechanisms of EGCG in 3T3-L1 cells. EGCG treatment down-regulates the expression of major genes involved in the adipogenesis pathway including peroxisome proliferator-activated receptor (PPAR)γ, CCAAT/enhancer binding protein (C/EBP)α, fatty acid binding protein (FABP)4 and fatty acid synthase (FASN), while up-regulating the nuclear level of β-catenin. Knockdown of β-catenin using small interfering (si) RNA attenuated the inhibitory effects of EGCG on intracellular lipid accumulation. β-catenin siRNA transfection also recovered terminal adipocyte markers such as FABP4, FASN, lipoprotein lipase and adiponectin, which were down-regulated by EGCG. The DNA binding activities as well as the expression levels of PPARγ and C/EBPα, which were down-regulated by EGCG, were significantly restored by β-catenin siRNA transfection. In addition, we found that EGCG efficiently up-regulates the WNT/β-catenin pathway. Among the members of the WNT/β-catenin pathway, the expressions of low density lipoprotein receptor-related protein (LRP)5, LRP6, disheveled (DVL)2 and DVL3 were significantly up-regulated, while AXIN expression was down-regulated by EGCG, and the phosphorylation of glycogen synthase kinase 3β was increased. These results suggest that EGCG activates the WNT/β-catenin pathway, resulting in the up-regulation of β-catenin, which down-regulates the major genes of the adipogenesis pathway. Taken together, our findings clearly show that the anti-adipogenic effects of EGCG are, at least partially, dependent on the WNT/β-catenin pathway.
Genistein, EGCG, and capsaicin Inhibit Adipocyte Differentiation process via activating AMP-activated protein kinase
Phytochemicals such as soy isoflavone genistein have been reported to possess therapeutic effects for obesity, diabetes, and cardiovascular diseases. In the present study, the molecular basis of selective phytochemicals with emphasis on their ability to control intracellular signaling cascades of AMP-activated kinase (AMPK) responsible for the inhibition of adipogenesis was investigated. Recently, the evolutionarily conserved serine/threonine kinase, AMPK, emerges as a possible target molecule of anti-obesity. Hypothalamic AMPK was found to integrate nutritional and hormonal signals modulating feeding behavior and energy expenditure.
We have investigated the effects of genistein, EGCG, and capsaicin on adipocyte differentiation in relation to AMPK activation in 3T3-L1 cells. Genistein (20-200muM) significantly inhibited the process of adipocyte differentiation and led to apoptosis of mature adipocytes. Genistein, EGCG, and capsaicin stimulated the intracellular ROS release, which activated AMPK rapidly. We suggest that AMPK is a novel and critical component of both inhibition of adipocyte differentiation and apoptosis of mature adipocytes by genistein or EGCG or capsaicin further implying AMPK as a prime target of obesity control.
Anti–Obesity effect of EGCG and glucosamine-6-phosphate through decreased expression of genes related to Adipogenesis and cell cycle arrest in 3T3-L1
Fatty acid synthase (FAS) has been recognized as a potential therapeutic target for Obesity. In this study, for the first time, the Inhibitory effect of pomegranate husk extract, punicalagin and ellagic acid on FAS was investigated. We found them potently Inhibiting the activity of FAS with half-Inhibitory concentration values (IC50) of 4.1 μg/ml (pomegranate husk extract), 4.2 μg/ml (4.50 μM, punicalagin) and 1.31 μg/ml (4.34 μM, ellagic acid), respectively. Moreover, they all exhibited time-dependent inactivation of FAS.
Punicalagin and ellagic acid Inhibited FAS with different mechanisms compared to previously reported Inhibitors, through inactivating acetyl/malonyl transferase and β-ketoacyl synthase domains, respectively. Additionally, 100 μg/ml pomegranate husk extract, 5.24 μg/ml (5 μM) punicalagin and 4.5 μg/ml (15 μM) ellagic acid effectively Reduced lipid accumulation inside FAS over-expressed 3T3-L1 Adipocytes. Since FAS plays a key role in the biosynthesis pathway of fatty acid, these findings suggest that pomegranate husk extract, punicalagin and ellagic acid have potential in the prevention and treatment of Obesity.
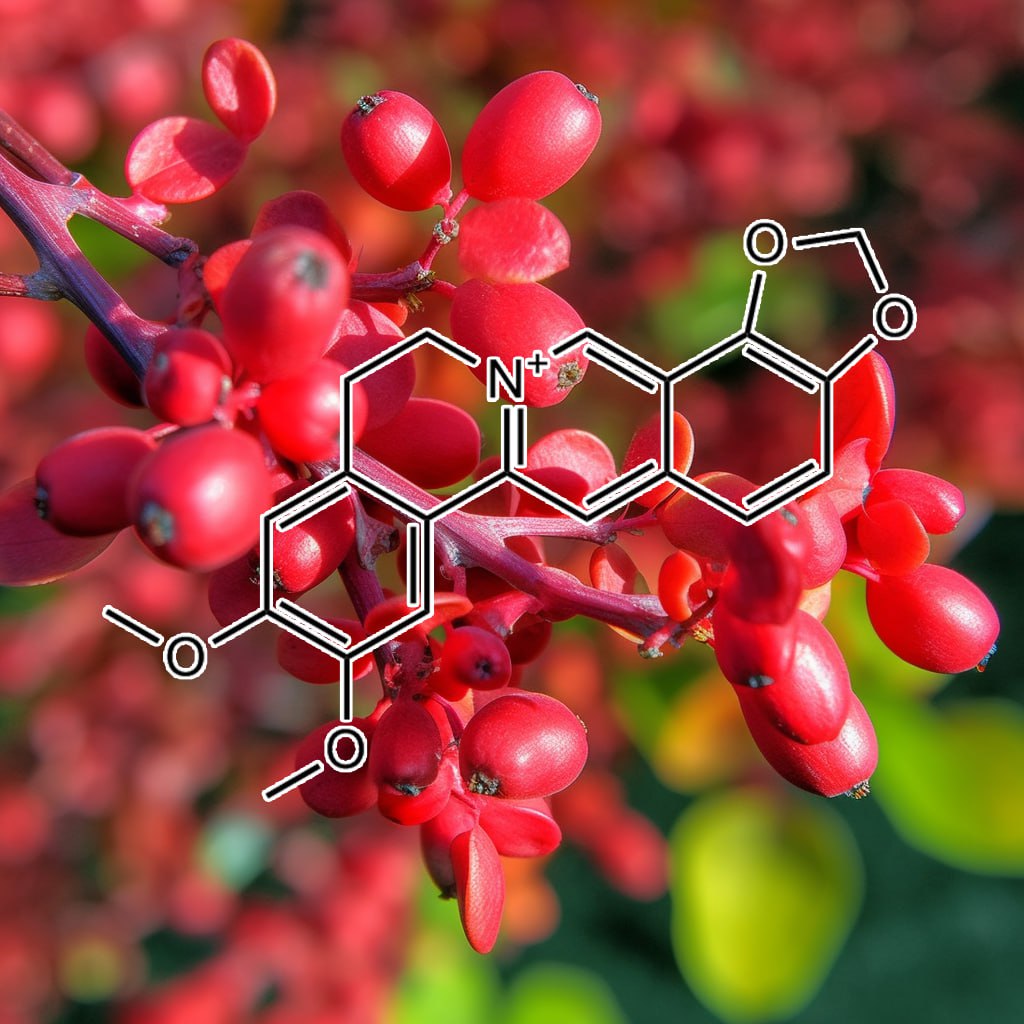
It has been reported that alkaloids derived from Coptis chinensis exert Anti-Adipogenic activity on 3T3-L1 Adipocytes by downregulating peroxisome proliferation-activity receptor-γ (PPAR-γ) and CCAAT/enhancer binding protein-α (C/EBP-α). However, the signaling-based mechanism of the Inhibitory role of epiberberine in the early stages of 3T3-L1 Adipocyte Differentiation is uncharacterized. Here, we show that epiberberine had Inhibitory effects on Adipocyte Differentiation and significantly decreased lipid accumulation by downregulating an Adipocyte -specific transcription factor, sterol regulatory element-binding protein-1 (SREBP-1).
Furthermore, we observed that epiberberine markedly suppressed the Differentiation -mediated phosphorylation of components of both the Raf/mitogen-activated protein kinase 1 (MEK1)/extracellular signal-regulated protein kinase 1/2 (ERK1/2) and AMP-activated protein kinase-α1 (AMPKα)/Akt pathways. In addition, gene expression of fatty acid synthase (FAS) was significantly Inhibited by treatment with epiberberine during Adipogenesis . These results indicate that the Anti-Adipogenic mechanism of epiberberine is associated with Inhibition of phosphorylation of Raf/MEK1/ERK1/2 and AMPKα/Akt, followed by downregulation of the major transcription factors of Adipogenesis , such as PPAR-γ, C/EBP-α, and SREBP-1, and FAS. Taken together, this study suggests that the Anti-Adipogenic effect of epiberberine is mediated by downregulation of the Raf/MEK1/ERK1/2 and AMPKα/Akt pathways during 3T3-L1 Adipocyte Differentiation . Moreover, the Anti-Adipogenic effects of epiberberine were not accompanied by modulation of β-catenin.

Obesity is one of the major public health problems in the world because it is implicated in Metabolic Syndrome s, such as type 2 Diabetes , hypertension, and cardiovascular diseases. The objective of this study was to investigate whether Erigeron annuus (L.) Pers. (EAP) extract Suppresses reactive oxygen species (ROS) production and Fat Accumulation in 3T3-L1 cells by activating an AMP-dependent kinase (AMPK) signaling pathway. Our results showed that EAP water extract significantly Inhibits ROS production, Adipogenesis , and lipogenesis during Differentiation of 3T3-L1 preadipocytes . In addition, EAP decreased mRNA and protein levels of proliferator-activated receptor γ (PPARγ ) and CCAAT/enhancer-binding protein alpha (C/EBPα ). Moreover, EAP suppressed mRNA expressions of fatty acid synthase (FAS), lipoprotein lipase (LPL), Adipocyte protein 2 (aP2) in a dose-dependent manner. Whereas, EAP upregulated adiponectin expression, phosphorylation levels of AMPK and carnitine palmitoyltransferase 1 (CPT-1) protein level during Differentiation of 3T3-L1 preadipocytes.
These results suggest that EAP water extract can exert ROS-linked Anti-Obesity effect through the mechanism that might involve Inhibition of ROS production, Adipogenesis and lipogenesis via an activating AMPK signaling pathway.
Objective: To determine the effects of esculetin, a plant phenolic compound with apoptotic activity in cancer cells, on 3T3‐L1 Adipocyte apoptosis and Adipogenesis .
Research Methods and Procedures: 3T3‐L1 pre‐confluent preadipocytes and lipid‐filled Adipocytes were incubated with esculetin (0 to 800 μM) for up to 48 hours. Viability was determined using the Cell Titer 96 Aqueous One Solution cell proliferation assay; apoptosis was quantified by measurement of single‐stranded DNA. Post‐confluent preadipocytes were incubated with esculetin for up to 6 days during maturation. Adipogenesis was quantified by measuring lipid content using Nile Red dye; cells were also stained with Oil Red O for visual confirmation of effects on lipid accumulation.
Results: In mature Adipocytes , esculetin caused a time‐ and dose‐related increase in Adipocyte apoptosis and a decrease in viability. Apoptosis was increased after only 6 hours by 400 and 800 μM esculetin (p < 0.05), and after 48 hours, as little as 50 μM esculetin increased apoptosis (p < 0.05). In preadipocytes , apoptosis was detectable only after 48 hours (p < 0.05) with 200 μM esculetin and higher concentrations. However, results of the cell viability assay indicated a reduction in Preadipocyte number in a time‐ and dose‐related manner, beginning as early as 6 hours with 400 and 800 μM esculetin (p < 0.05). Esculetin also Inhibited Adipogenesis of 3T3‐L1 preadipocytes . Esculetin‐mediated Inhibition of Adipocyte Differentiation occurred during the early, intermediate, and late stages of the Differentiation process. In addition, esculetin induced apoptosis during the late stage of Differentiation .
Discussion: These findings suggest that esculetin can alter fat cell number by direct effects on cell viability, Adipogenesis, and apoptosis in 3T3‐L1 cells.
Euphorbia lunulata
The insulin-like and/or insulin-sensitising effects of Syzygium aqueum leaf extract and its six bioactive compounds; 4-hydroxybenzaldehyde, myricetin-3-O-rhamnoside, europetin-3-O-rhamnoside, phloretin, myrigalone-G and myrigalone-B were investigated in 3T3-L1 Adipocytes. We observed that, S. aqueum leaf extract (0.04–5 μg/ml) and its six bioactive compounds (0.08–10 μM) at non-cytotoxic concentrations were effectively enhance Adipogenesis, stimulate glucose uptake and increase adiponectin secretion in 3T3-L1 Adipocytes. Clearly, the compounds myricetin-3-O-rhamnoside and europetin-3-O-rhamnoside showed insulin-like and insulin-sensitising effects on Adipocytes from a concentration of 0.08 μM.
These compounds were far better than rosiglitazone and the other isolated compounds in enhancing Adipogenesis, stimulating 2-NBDG uptake and increasing adiponectin secretion at all the concentrations tested. These suggest the AntiDiabetic potential of S. aqueum leaf extract and its six bioactive compounds. However, further molecular interaction studies to explain the mechanisms of action are highly warranted.
Evodiamine (Evodia rutaecarpa)
Evodiamine Inhibits Adipogenesis via the EGFR–PKCα–ERK signaling pathway
The molecular mechanism of the Anti-Adipogenic effect of evodiamine (which has several capsaicin-like pharmacological actions) was investigated. The evodiamine effect was not blocked by the specific TRPV1 antagonist capsazepine in 3T3-L1 preadipocytes , whereas its effect was greatly curtailed by Inhibitors of protein kinase C (PKC) and epidermal growth factor receptor (EGFR). Signal analyses showed that evodiamine stimulated the phosphorylation of EGFR, PKCα, and ERK, all of which were Reduced by an Inhibitors EGFR Inhibitor.
Silencing experiments of EGFR mRNA supported the involvement of these signaling molecules in the Inhibitory effect of evodiamine. An unidentified mechanism whereby evodiamine Inhibits Adipogenesis via the EGFR–PKCα–ERK signaling pathway was revealed.
Ferulic Acid (Ferula assafoetida L)
World epidemic Obesity is a major contributing factor to metabolic diseases including insulin resistance and cardiovascular diseases. In this study, aqueous and ethanolic extracts of hulled barley with roasting temperatures of up to 250 °C were investigated for their Anti-Adipogenic effects in vitro and in vivo. An aqueous extract of hulled barley roasted at 210 °C (AHB210) effectively Inhibited Adipocyte Differentiation. Intraperitoneal injections of 15 or 50 mg/kg AHB210 dose dependently prevented body Weight Gain, fat Mass increase, and dysregulated lipid profiles in high fat diet-induced obese male mice. In addition, oral administration of AHB210 to ovariectomized rats also prevented body Weight Gain.
A high performance liquid chromatographic analysis identified coumaric acid and ferulic acid as primary Anti-Obesity mediators. The presence of beta glucan in AHB210 was less likely to be responsible for the lipid accumulating actions. Taken together, AHB210 may be useful to Prevent Obesity and its related metabolic diseases.
Ficus deltoidea
Objective: This study examined the Anti-Adipogenic effects of extracts of Ficus deltoidea var. deltoidia and var. angustifolia, a natural slimming aid, on 3T3-L1 Adipocytes.
Methods: Methanol and water extracts of leaves of the F. deltoidea varieties were analyzed to determine their total flavonoid content (TFC) and total phenolic content (TPC), respectively. The study was initiated by determining the maximum non-toxic dose (MNTD) of the methanol and water extracts for 3T3-L1 preadipocytes. Possible Anti-Adipogenic effects were then examined by treating 2-d post confluent 3T3-L1 preadipocytes with either methanol extract or water extract at MNTD and half MNTD (½MNTD), after which the preadipocytces were induced to form mature Adipocytes. Visualisation and quantification of lipid content in mature Adipocytes were carried out through oil red O staining and measurement of optical density (OD) at 520 nm, respectively.
Results: The TFCs of the methanol extracts were 1.36 and 1.97 g quercetin equivalents (QE)/100 g dry weight (DW), while the TPCs of the water extracts were 5.61 and 2.73 g gallic acid equivalents (GAE)/100 g DW for var. deltoidea and var. angustilofia, respectively. The MNTDs determined for methanol and water extracts were (300.0±28.3) and (225.0±21.2) µg/ml, respectively, for var. deltoidea, while much lower MNTDs [(60.0±2.0) µg/ml for methanol extracts and (8.0±1.0) µg/ml for water extracts] were recorded for var. angustifolia. Studies revealed that the methanol extracts of both varieties and the water extracts of var. angustifolia at either MNTD or ½MNTD significantly Inhibited the maturation of preadipocytes.
Conclusions: The Inhibition of the formation of mature Adipocytes indicated that leaf extracts of F. deltoidea could have potential Anti-Obesity effects.
fisetin (Cotinus Coggygria)
Adipocytes are the key player in Adipose Tissue Inflammation and subsequent systemic insulin resistance and its development involves complex process of proliferation and Differentiation of preadipocytes. Fistein, a Polyphenol flavonoid, is known to exert anti-inflammatory, anti-carcinogenic and anti-Diabetic effects. In this study, we aimed to investigate the effect of fisetin on Adipocyte proliferation and Differentiation in 3T3-L1 Preadipocyte cell line and its mechanism of action. We found that fisetin Inhibits Adipocyte Differentiation in a concentration dependent manner, which were evidenced by Oil Red O staining and the protein expression of mature Adipocyte marker genes fatty acid synthase and peroxisome proliferator-activated receptor γ.
Moreover, the proliferation of preadipocytes was also markedly suppressed by treatment of fisetin for 24 and 48 h in the Differentiation medium. We also found that fisetin Inhibition of Adipocyte Differentiation was largely due to the effect on mitotic clonal expansion. Fisetin suppression of Preadipocyte proliferation at early stage of Differentiation was accompanied by the changes of expression of a series of cell cycle regulatory proteins. Altogether, our results suggest that the Inhibition of Adipocyte Differentiation by fisetin may be at least in part mediated by cell cycle arrest during Adipogenesis.
Foenumoside B (Lysimachia foenum-graecum)
We have previously reported anti-obesity effects of Lysimachia foenum-graecum in high-fat diet (HFD)-induced obesity model. Here we isolated a triterpene saponin foenumoside B as an active component of L. foenum-graecum. Foenumoside B blocked the differentiation of 3T3-L1 preadipocytes in a dose-dependent manner with an IC50 of 0.2 μg/ml in adipogenesis assay and suppressed the induction of PPARγ, the master regulator of adipogenesis. Foenumoside B induced the activation of AMP-activated protein kinase (AMPK), and modulated the expression of genes involved in lipid metabolism towards lipid breakdown in differentiated adipocytes. In mouse model, oral administration of foenumoside B (10mg/kg/day for 6 weeks) reduced HFD-induced body weight gain significantly without affecting food intake. Treatment of foenumoside B suppressed lipid accumulation in white adipose tissues and the liver, and lowered blood levels of glucose, triglycerides, ALT, and AST in HFD-induced obese mice.
Consistent with the in vitro results, foenumoside B activated AMPK signaling, suppressed the expression of lipogenic genes, and enhanced the expression of lipolytic genes in vivo. Foenumoside B also blocked HFD-induced proinflammatory cytokine production in adipose tissue, suggesting its protective role against insulin resistance. Taken together, these findings demonstrate that foenumoside B represents the anti-obesity effects of L. foenum-graecum, and suggest therapeutic potential of foenumoside B in obesity and obesity-related metabolic diseases.
Suppression of Adipocyte Differentiation by Foenumoside B from Lysimachia foenum–graecum Is Mediated by PPARγ Antagonism
Lysimachia foenum-graecum extract (LFE) and its active component foenumoside B (FSB) have been shown to inhibit adipocyte differentiation, but their mechanisms were poorly defined. Here, we investigated the molecular mechanisms responsible for their anti-adipogenic effects. Both LFE and FSB inhibited the differentiation of 3T3-L1 preadipocytes induced by peroxisome proliferator-activated receptor-γ (PPARγ) agonists, accompanied by reductions in the expressions of the lipogenic genes aP2, CD36, and FAS. Moreover, LFE and FSB inhibited PPARγ transactivation activity with IC50s of 22.5 μg/ml and 7.63 μg/ml, respectively, and showed selectivity against PPARα and PPARδ. Rosiglitazone-induced interaction between PPARγ ligand binding domain (LBD) and coactivator SRC-1 was blocked by LFE or FSB, whereas reduced NCoR-1 binding to PPARγ by rosiglitazone was reversed in the presence of LFE or FSB.
In vivo administration of LFE into either ob/ob mice or KKAy mice reduced body weights, and levels of PPARγ and C/EBPα in fat tissues. Furthermore, insulin resistance was ameliorated by LFE treatment, with reduced adipose tissue inflammation and hepatic steatosis. Thus, LFE and FSB were found to act as PPARγ antagonists that improve insulin sensitivity and metabolic profiles. We propose that LFE and its active component FSB offer a new therapeutic strategy for metabolic disorders including obesity and insulin resistance.
Balance between Adipocyte and osteoblast Differentiation is the key link of disease progression in Obesity and osteoporosis. We have previously reported that formononetin (FNT), an isoflavone extracted from Butea monosperma, stimulates osteoblast formation and protects against postmenopausal bone loss. The inverse relationship between osteoblasts and Adipocytes prompted us to analyse the effect of FNT on Adipogenesis and in vivo bone loss, triggered by high-fat diet (HFD)-induced Obesity. The Anti-Obesity effect and mechanism of action of FNT was determined in 3T3-L1 cells and HFD-induced obese male mice. Our findings show that FNT Suppresses the Adipogenic Differentiation of 3T3-L1 fibroblasts, through down-regulation of key Adipogenic markers such as PPARγ, CCAAT/enhancer-binding protein alpha (C/EBPα) and sterol regulatory element-binding protein (SREBP) and Inhibits intracellular TAG accumulation. Increased intracellular reactive oxygen species levels and AMP-activated protein kinase (AMPK) activation accompanied by stabilisation of β-catenin were attributed to the Anti-Adipogenic action of FNT. In vivo, 12 weeks of FNT treatment Inhibited the development of Obesity in mice by attenuating HFD-induced body Weight Gain and visceral Fat Accumulation. The Anti-Obesity effect of FNT results from increased energy expenditure.
FNT also protects against HFD-induced dyslipidaemia and rescues deterioration of trabecular bone volume by increasing bone formation and decreasing bone resorbtion caused by HFD. FNT’s rescuing action against Obesity-induced osteoporosis commenced at the level of progenitors, as bone marrow progenitor cells, obtained from the HFD mice group supplemented with FNT, showed increased osteogenic and decreased Adipogenic potentials. Our findings suggest that FNT Inhibits Adipogenesis through AMPK/β-catenin signal transduction pathways and protects against HFD-induced Obesity and bone loss.
fucoxanthin
Fucoxanthin and its metabolite, fucoxanthinol, suppress Adipocyte Differentiation in 3T3-L1 cells
Fucoxanthin is a major carotenoid found in edible seaweed such as Undaria pinnatifida and Hijikia fusiformis. We investigated the suppressive effects of fucoxanthin and its metabolite, fucoxanthinol, on the differentiation of 3T3-L1 preadipocytes to adipocytes. Fucoxanthin inhibited intercellular lipid accumulation during adipocyte differentiation of 3T3-L1 cells. Furthermore, fucoxanthin was converted to fucoxanthinol in 3T3-L1 cells. Fucoxanthinol also exhibited suppressive effects on lipid accumulation and decreased glycerol-3-phosphate dehydrogenase activity, an indicator of adipocyte differentiation.
The suppressive effect of fucoxanthinol was stronger than that of fucoxanthin. In addition, in 3T3-L1 cells treated with fucoxanthin and fucoxanthinol, peroxisome proliferator-activated receptor gamma (PPARgamma), which regulates adipogenic gene expression, was down-regulated in a dose-dependent manner. These results suggest that fucoxanthin and fucoxanthinol inhibit the adipocyte differentiation of 3T3-L1 cells through down-regulation of PPARgamma. Fucoxanthinol had stronger suppressive effects than fucoxanthin on adipocyte differentiation in 3T3-L1 cells.
Fucoxanthin and its deacetylated metabolite fucoxanthinol are two major carotenoids that have been confirmed to possess various pharmacological properties. In the present study, fucoxanthinol was identified as the deacetylated metabolite of fucoxanthin, after intravenous (i.v.) and intragastric gavage (i.g.) administration to rats at doses of 2 and 65 mg/kg, respectively, by liquid chromatography-tandem mass spectrometric (LC-MS/MS) analysis. Next, an accurate and precise LC-MS/MS method was developed to quantitatively determine fucoxanthin and fucoxanthinol in rat plasma.
Plasma samples were resolved by LC-MS/MS on a reverse-phase SB-C18 column that was equilibrated and eluted with acetonitrile (A)/aqueous 0.1% formic acid (B; 92/8, v/v) at a flow rate of 0.5 mL/min. Analytes were monitored by multiple-reaction monitoring (MRM) under positive electrospray ionization mode. The precursor/product transitions (m/z) were 659.3→109.0 for fucoxanthin, 617.2→109.0 for fucoxanthinol, and 429.4→313.2 for the internal standard (IS). Calibration curves for fucoxanthin and fucoxanthinol were linear over concentrations ranging from 1.53 to 720 and 1.17 to 600 ng/mL, respectively. The inter- and intraday accuracy and precision were within ±15%. The method was applied successfully in a pharmacokinetic study and the resulting oral fucoxanthin bioavailability calculated.
Gallotannin (Mangifera indica)
Expansion of adipose tissue in obesity is associated with dysregulation of adipokines, which can lead to long-term metabolic disorders. Gallotannin derivatives from mango possess anti-inflammatory activities, but their potential role in lipid metabolism is not well investigated. In this study, 3T3-L1 preadipocytes were differentiated into adipocytes and treated with mango polyphenols (MG), or pyrogallol (PG) for 6 days. The anti-adipogenic activity of PG was demonstrated by reduced number of lipid droplets and expressions of adipogenic markers, such as peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein α (C/EBPα).
In mature adipocytes, PG promoted adipocyte browning and increased the expressions of uncoupling protein 1 (UCP1) and sirtuin 1 (Sirt1). Knockdown of AMP-activated protein kinase (AMPK) α1 with siRNA partially abolished the effect of PG on adipogenesis. Results indicate that gallotannin derivatives modulate lipid metabolism, at least in part, though the AMPK pathway, and possess potential to prevent obesity-related conditions.
Mango supplementation improved the plasma levels of proinflammatory cytokines and metabolic hormones in obese participants partly due to increased systemic exposure to polyphenolic metabolites. In summary, health benefits of mango-derived polyphenols in obesity and insulin resistance are mainly attributed to the production of microbial metabolites of GT, which is in part through the interactions with the AMPK-C/EBPα/PPARγ and AMPKUCP1/Sirt1 axes in adipose tissue. Improving the abundance of probiotics in gut microbiota may improve the bioavailability of mango-derived polyphenols, resulting in enhanced efficacy of the microbial metabolites in the prevention of lipid accumulation and metabolic dysfunction in obesity.

Four new lanostane triterpenes, butyl ganoderate A, butyl ganoderate B, butyl lucidenate N, and butyl lucidenate A, were isolated from the fruiting bodies of Ganoderma lucidum together with 14 known compounds. The structures of the new triterpenes were established by extensive spectroscopic studies and chemical evidence. In addition, the Inhibitory effect of isolated compounds on Adipocyte Differentiation in 3T3-L1 cells was examined.

Gelidium elegans
Previously, we showed that Gelidium elegans extract (GE) Suppresses oxidative stress and lipid accumulation. However, the molecular mechanism underlying the Anti-Adipogenic ability of GE is still unclear. The levels of Adipogenesis markers and triglyceride synthesis enzymes were measured by western blot. To evaluate the lipid accumulation in 3T3-L1 cells, oil red o staining was performed. We investigated whether GE induces Lipolysis by measuring Adipocyte triglyceride lipase (ATGL) during Adipocyte Differentiation. We also examined the expression of beige cell-associated genes and the production of carbon dioxide in 3T3-L1 cells. We showed that GE increased the protein expression of CAAT/enhancer binding protein (C/EBP) homologous protein 10 and Inhibited the expression of C/EBPβ. GE discouraged triglyceride synthesis via deregulation of lysophosphatidic acid acyltransferase-θ (LPAATθ) and diacylglycerolacyltransferase 1 (DGAT1) during late-stage Adipogenesis in 3T3-L1 cells. GE also dramatically increased ATGL in 3T3-L1 cells.
Finally, in 3T3-L1 cells treated with GE, markers of beige Adipocytes such as PRDM16 and UCP1 were upregulated, and large amounts of carbon dioxide were produced. These data indicate that GE Suppresses Adipogenesis by stimulating a beige-like phenotype in 3T3-L1 cells.
Genistein
genistein’s Anti-Adipogenic effect coincides with the expression of C\EBPβ, C\EBPα and PPARγ , we hypothesized that genistein Inhibits 3T3-L1 cell …
Microarrays showed that anti‐Adipogenic effects of genistein were principally attributable to activation of Wnt signalling via ERs‐dependent pathway, such as Erk/JNK signalling and LEF/TCF4 co … Unlike genistein, daidzein Inhibited Adipogenesis through stimulation of …
The AntiAdipogenic effect of genistein was not due to Inhibition of insulin receptor subtrate-1 …Genistein (4′,5,7-trihydroxyisoflavone) and all tissue culture materials were from GIBCO … Rabbit polyclonal anti-signal transducers and activators of transcription (STAT) 3 antibody was …
Compounds genistein (G), quercetin (Q), and resveratrol (R) have been reported to each exhibit Anti-Adipogenic activities in Adipocytes and antiproliferative and pro-apoptotic activities in several cell types. We studied the combined effects of G, Q, and R on Adipogenesis.
At d 3 of Adipogenesis , TGFβ1 was strongly up-regulated by genistein in an ER-dependent manner …Adipogenic Differentiation and maturation, on the other hand, were Reduced by genistein (and 17β-estradiol) via an ER-dependent mechanism involving autocrine or …
Gentiopicroside (Gentiana scabra)
Ginkgetin (Ginkgo biloba)
Adipogenesis involved in hypertrophy and hyperplasia of Adipocytes is responsible for expanding the Mass of Adipose Tissue s in obese individuals. Peroxisome proliferator-activated receptor γ (PPARγ ) and CCAAT/enhancer-binding protein α(C/EBPα ) are two principal transcription factors induced by delicate signaling pathways, including signal transducer and activator of transcription 5 (STAT5), in Adipogenesis. Here, we demonstrated a novel role of ginkgetin, a biflavone from Ginkgo biloba leaves, as a STAT5 Inhibit or that blocks the Differentiation of preadipocytes into Adipocytes. During the Differentiation of 3T3-L1 cells, ginkgetin treatment during the first 2 days markedly Inhibited the formation of lipid-bearing Adipocytes. PPARγ and C/EBPα expression was decreased in 3T3-L1 cells during Adipogenesis following ginkgetin treatment, whereas no change was observed in C/EBPβ or C/EBPδ expression. Inhibition of PPARγ and C/EBPα expression by ginkgetin occurred through the prevention of STAT5 activation during the initiation phase of Adipogenesis.
In addition, ginkgetin-mediated the Inhibition of Adipogenesis was recapitulated in the Differentiation of primary preadipocytes. Lastly, we confirmed the Inhibitory effects of ginkgetin on the hypertrophy of white Adipose Tissues from high-fat diet-fed mice. These results indicate that ginkgetin is a potential anti-Adipogenesis and Anti-Obesity drug.
Ginkgetin: A natural biflavone with versatile pharmacological activities
Natural products, being richly endowed with curative powers, have become spotlight for biomedical and pharmaceutical research to develop novel therapeutics during recent years. Ginkgetin, a natural non-toxic biflavone, has been shown to exhibit anti-cancer, anti-inflammatory, anti-microbial, Anti-Adipogenic , and neuroprotective activities. Ginkgetin combats cancer progression by arresting cell cycle, inducing apoptosis, stimulating autophagy, and targeting many deregulated signaling pathways such as JAK/STAT and MAPKs. Ginkgetin halts Inflammation mediators like interleukins, iNOS, COX-2, PGE2, NF-κB, and acts as an Inhibit or of PLA2. GK shows strong neuroprotection against oxidative stress-promoted cell death, Inhibits cerebral micro-hemorrhage, decreases neurologic deficits, and halts apoptosis of neurons.
In conclusion, isoginkgetin is a promising candidate compound for the treatment of insulin resistance based on our in vitro studies. Further in vivo studies are warranted. Analogs of isoginkgetin possessing higher potency in adiponectin production and lower PPARγ agonist activity can also be explored to identify more active compounds in vivo. Currently, insulin-sensitizing drugs mainly include PPARγ agonists and compounds that target the insulin signaling pathway. This study presents for the first time a potential new strategy for the discovery of insulin sensitizers by screening adiponectin production. Moreover, the critical genes involved in isoginkgetin action may provide novel targets for anti-Diabetic therapy.
GbE may have a potentially therapeutic use for menopause-associated Obesity; supplementation with 500 mg/kg of GbE stimulated hypothalamic serotonergic activity in ovariectomized rats (28). GbE isolated bioactive compounds have been demonstrated to stimulate Lipolysis in 3T3-L1 Adipocytes (29), and to Inhibit Adipogenesis through activation of the AMPK pathway (30). However, the effects of GbE supplementation on metabolic processes of visceral Adipose Tissue in DIO rats remain largely unknown. In view of the considerations highlighted above, the aim of the present study was to investigate the effects of GbE supplementation as a potentially anti-obesogenic effector for improvement in lipid metabolism of epididymal Adipose Tissue of DIO rats.
Ginkgo Biloba
Adipogenesis involved in hypertrophy and hyperplasia of Adipocytes is responsible for expanding the Mass of Adipose Tissue s in obese individuals. Peroxisome proliferator-activated receptor γ (PPARγ ) and CCAAT/enhancer-binding protein α (C/EBPα ) are two principal transcription factors induced by delicate signaling pathways, including signal transducer and activator of transcription 5 (STAT5), in Adipogenesis. Here, we demonstrated a novel role of ginkgetin, a biflavone from Ginkgo biloba leaves, as a STAT5 Inhibit or that blocks the Differentiation of preadipocytes into Adipocytes.
During the Differentiation of 3T3-L1 cells, ginkgetin treatment during the first 2 days markedly Inhibited the formation of lipid-bearing Adipocytes . PPARγ and C/EBPα expression was decreased in 3T3-L1 cells during Adipogenesis following ginkgetin treatment, whereas no change was observed in C/EBPβ or C/EBPδ expression. Inhibition of PPARγ and C/EBPα expression by ginkgetin occurred through the prevention of STAT5 activation during the initiation phase of Adipogenesis. In addition, ginkgetin-mediated the Inhibition of Adipogenesis was recapitulated in the Differentiation of primary preadipocytes. Lastly, we confirmed the Inhibitory effects of ginkgetin on the hypertrophy of white Adipose Tissues from high-fat diet-fed mice. These results indicate that ginkgetin is a potential anti-Adipogenesis and Anti-Obesity drug.
Ginsenoside Rg1, Rg2, Rg3
Rg1 exhibits an Anti-Adipogenic effect via regulation of the expression of the transcriptional factors and lipid metabolism-related genes in vivo and in vitro. We observed that Rg1 administration significantly increased the phosphorylation level of AMP-activated protein kinase (AMPK) in both epididymal white Adipose Tissue and 3T3-L1 cells. These results indicated that Rg1 works both in an Anti-Adipogenic and Anti-Obesity manner through inducing AMPK activation, Inhibiting lipogenesis, and decreasing intracellular lipid content, Adipocyte size, and adipose weight.
Anti–Adipogenic effects and mechanisms of ginsenoside Rg3 in Pre-Adipocytes and obese mice
Red or black ginseng has been reported more powerful than white/fresh ginseng in dealing with various diseases/conditions including Obesity. The major reason is that heating/steaming, the process of making red or black ginseng, produces large amount of bioactive compounds including ginsenoside Rg3 (Rg3), which are trace in fresh or white ginseng. In the present study, Rg3 was applied both in Pre-Adipocytes and obese mice to investigate the Anti-Adipogenic effects and relevant mechanisms. Our results show that Rg3 dose-dependently Inhibited cell Differentiation both in 3T3-L1 cells (30, 50, and 100 μM) and human primary Pre-Adipocytes (10, 20, and 30 μM).
This Inhibitory effect is accompanied by the attenuation of the expressions of Adipogenic markers including peroxisome proliferator-activated receptor gamma (PPAR-γ), CCAAT/enhancer binding protein alpha (C/EBP-α), fatty acid synthase (FAS), fatty acid binding protein 4 (FABP4) and perilipin. Although dietary intake of Rg3 (0.1 mg Rg3/kg diet, 8 weeks) did not significantly affect body Weight Gain , fat pads and food intake as well as of PPAR-γ expression in fat tissues, we found that hepatic PPAR-γ and C/EBP-α protein expressions and hepatic glutathione reductase and glutathione S-transferase, two major Antioxidants molecules were significantly Reduced by Rg3. These results suggest that ginsenoside Rg3 may be a potential agent in reducing/preventing Obesity.
AntiObesity effect of ginsenoside Rg3 involves the AMPK and PPAR‐γ signal pathways
Ginsenosides, the active component of ginseng, exerts AntiDiabetic and anticancer effects. This study investigated the molecular basis of ginsenoside Rg3, a red ginseng rich constituent, focusing on its ability to Inhibit Adipocyte Differentiation in 3T3‐L1 cells. The data show that ginsenoside Rg3 was effective in the Inhibition of Adipocyte Differentiation.
This Inhibitory effect of ginsenoside Rg3 on Adipocyte Differentiation was accompanied by PPAR‐γ Inhibition in rosiglitazone‐treated cells. The study also tested whether AMP‐activated protein kinase (AMPK) activation was involved in the Inhibitory effects of ginsenoside Rg3. AMPK plays a role in maintaining health in the context of diseases such as type 2 Diabetes, Obesity and cancer. AMPK was reported to control nutritional and hormonal signal modulating. Rg3 significantly and time‐dependently activated AMPK. Taken together, these results suggest that the AntiObesity effect of red ginseng rich constituent, ginsenoside Rg3, involves the AMPK signaling pathway and PPAR‐γ Inhibition.
Ginsenoside Rg3 ameliorated HFD-induced hepatic steatosis through downregulation of STAT5-PPARγ
The LC50 value of Rg3 in 3T3-L1 cell lipogenesis is 200 μM, and our working concentrations (5, 25 and 50 μM) of Rg3 Reduced lipogenesis without cell toxicity (Fig. 1B and C). To investigate the Inhibiting effect on lipid accumulation by Rg3, we treated 3T3-L1 cells for 3 or 9 days. Oil Red O staining confirmed lipid accumulation in the cell. At 3 days, the lipogenic cocktail MDI (insulin, dexamethasone and isobutylmethylxanthine) induced in Pre-Adipocytes a very low increase in their accumulated fat deposits. When Rg3 was combined with MDI-induced cells, their lipid accumulation was suppressed (Fig. 1D), in an Rg3 dose-dependent manner, as assessed at 9 days after treatment using Oil Red O staining microscopy data and Oil Red O absorbance at 520 nm (Fig. 1E). In Adipocytes, lipid accumulation is related to the amount of TGs in the cell. Similar to the Oil Red O staining results, TG storage was Reduced by Rg3 treatment in 3T3-L1 cells in a dose-dependent manner (Fig. 1F). These data suggest that Rg3 can Prevent lipogenesis and lipid accumulation in the 3T3-L1 cell line.
Ginsenoside Rg3 induces browning of 3T3-L1 Adipocytes by activating AMPK signaling
Rg3 induced the expression of beige fat-specific genes (CD137 and TMEM26) and lipid metabolism-associated genes (FASN, SREBP1, and MCAD), which indicated the activation of lipid metabolism by Rg3. We also demonstrated that activation of 5’ adenosine monophosphate-activated protein kinase (AMPK) is required for Rg3-mediated up-regulation of browning gene expression. Moreover, Rg3 Inhibited the accumulation of lipid droplets and Reduced the droplet size in mature 3T3-L1 Adipocytes.
Taken together, this study identifies a novel role of Rg3 in browning of white Adipocytes, as well as suggesting a potential mechanism of an Anti-Obesity effect of Panax ginseng.

Glehnia littoralis Root

Guarana (Paullinia cupana) is a plant originated in Brazil that presents a beneficial effect on body weight control and metabolic alterations. The aim of this study was to evaluate the effects of guarana on genes and miRNAs related to Adipogenesis in 3T3L1 cells. The Anti-Adipogenic effect of guarana was evaluated by Oil Red-O staining. Gene and miRNA expression levels were determined by real time PCR. The Cebpα and β-catenin nuclear translocation were evaluated using immunocytochemistry. Our data indicated that the triglyceride-reducing effect of guarana was dose-dependent from 100 to 300 µg/mL (−12%, −20%, −24% and −40%, respectively, p < 0.0001). An up-regulation of the Anti-Adipogenic genes Wnt10b, Wnt3a, Wnt1, Gata3 and Dlk1 and a down-regulation of Pro-Adipogenic genes Cebpα, PPARγ and Creb1 were also observed. Furthermore, guarana repressed mmu-miR-27b-3p, mmu-miR-34b-5p and mmu-miR-760-5p, that contributed for up-regulation of their molecular targets Wnt3a, Wnt1 and Wnt10b.
Results: In mature Adipocytes cis‐GS decreased viability, whereas the trans‐GS isomer had little effect. Both isomers caused dose‐dependent increases in apoptosis and cis‐GS was more effective than trans‐GS in inducing apoptosis. cis‐ and trans‐GS also increased caspase‐3 activity and release of cytochrome c from mitochondria. In maturing preadipocytes , both isomers were equally effective in reducing lipid content. The Adipocyte ‐specific transcription factors PPARγ 2, C/EBPα , and C/EBPβ were downregulated after treatment with cis‐GS during the maturation period. Furthermore, cis‐GS increased basal Lipolysis of mature Adipocytes , but trans‐GS had no effect.
Discussion: These results indicate that GS isomers may exert AntiObesity effects by Inhibiting Differentiation of preadipocytes , and by inducing apoptosis and promoting Lipolysis of mature Adipocytes . The cis‐GS isomer was more potent than the trans‐GS isomer in inducing apoptosis and Lipolysis in mature Adipocytes.

PME supplementation significantly up-regulated the PPARα, CPT1, CPT2, UCP1 and HSL mRNA levels compared with the HFD group, whereas it down-regulated expression of the PPARγ and DGAT2 genes. Finally, HFD increased serum leptin, insulin, glucose and insulin and glucose levels; however, PME reversed these changes. These results demonstrated that PME might relieve Obesity that occurs via Inhibition of Adipogenesis and lipogenesis as well as through Lipolysis and fatty acid oxidation in 3T3-L1 cells and HFD-induced obese mice.

We explored the potential of hesperidin and capsaicin, separately and in combination, to induce white Adipose Tissue (WAT) browning and to help body weight management in Western diet-fed rats. Adult male Wistar rats were fed for 8 weeks with Western diet and treated daily with hesperidin (100 mg/kg/day), capsaicin (4 mg/kg/day), hesperidin (100 mg/kg/day) + capsaicin (4 mg/kg/day), or the vehicle. Hesperidin and capsaicin separately, but not (or to a lesser extent) the combination, resulted in a decreased size of Adipocytes and induced emergence of multilocular brown-like Adipocytes positive for UCP1 and CIDEA in retroperitoneal WAT.
Expression levels of browning markers, such as Prdm16, in inguinal WAT also increased with capsaicin treatment compared with the vehicle (145% ± 17% vs 92% ± 21%, P < 0.05), but no significant effects were found with the combination (106% ± 12%). Thus, the combination of both bioactives Reduces the effectiveness of each compound to decrease the Adipocyte size and induce WAT browning.

Hibiscus rosa sinensis flower
AMPK activating and anti Adipogenic potential of Hibiscus rosa sinensis flower in 3T3-L1 cells
Results: Treatment with HRF 25 and 50 µg/mL activated AMP-activated protein kinase (AMPK) and was found to alleviate triglyceride accumulation significantly (p < 0.001) by 1.6 and 2.3 times respectively in pre adipocytes during differentiation. HRF 25 and 50 µg/mL also nonsignificantly reduced lipolysis which releases free fatty acids, a major contributing factor for insulin resistance. Activation of AMPK by phosphorylation has led to reduced gene and protein expression of adipogenic factors Peroxisome proliferator- activated receptor gamma (PPAR-γ), CCAT/enhancer binding protein alpha (C/EBPα), Sterol regulatory element- binding protein-1c (SREBP-1c) and their targets Fatty acid binding protein 4 (FABP4), Fatty acid synthase (FAS), Perilipin and enhanced Adiponectin expression. Treatment with HRF 25 and 50 µg/mL also resulted in inactivation of Acetyl-CoA carboxylase (ACC) by enhancing ACC phosphorylation, which reduced the levels of malonyl-CoA an allosteric inhibitor of carnitine palmitoyl transferase 1 (CPT1). Enhanced CPT1 levels causes induction of fatty acid β- oxidation. Effects of HRF were nullified in the presence of AMPK antagonist dorsomorphin.
Conclusion: In summary, HRF treatments reduced adipogenesis, enhanced factors regulating fatty acid oxidation and this is mediated by AMPK activation. The results conclusively showed anti-obesity potential of HRF and it might be helpful in treatment of associated complications.
Objectives: This study was designed to investigate the effect of hibiscus (Hibiscus sabdariffa) on adipogenic differentiation of 3T3-L1 cells at the cellular and molecular levels.
Design: Various concentrations of hibiscus extract were added to confluent 3T3-L1 preadipocytes at the outset of the differentiation program and further incubated for 36 hours. Cells were maintained in postdifferentiation medium containing insulin with hibiscus extract in complete culture medium.
Results: Hibiscus extract inhibited the adipocyte differentiation of 3T3-L1 preadipocytes induced by insulin, dexamethasone, and isobutylmethylxanthine (IBMX) in a dose-dependent manner. Hibiscus blocked the cytoplasmic lipid accumulation when administered at the onset of differentiation and 4 days after induction of differentiation. The inhibitory effect of hibiscus on adipogenic lipid accumulation of preadipocytes was significant (p < 0.01) between control cells and cells treated with hibiscus. Hibiscus extract significantly attenuated the expression of key adipogenic transcription factors, including CCAAT element binding protein (C/EBP)alpha and peroxisome proliferator-activated receptor (PPAR)gamma at protein levels.
Conclusion: These results suggest that hibiscus extract blocks adipogenesis, in part, by its suppression on the expression of adipogenic transcription factors, including C/EBPalpha and PPARgamma.
Hibiscus sabdariffa L
Beneficial effects of natural bioactive compounds from Hibiscus sabdariffa L. on Obesity
This review has gathered reports on the various anti-obesity effects of H. sabdariffa bioactive compounds in cell and animal models, as well as in humans. Available toxicology information on the consumption of H. sabdariffa revealed that its toxicity is dose-dependent and may cause an adverse effect when administered over a long period of time. Reports have shown that H. sabdariffa derived bioactive compounds are potent in the treatment of obesity with an evident reduction in body weight, inhibition of lipid accumulation and suppression of adipogenesis through the PPARγ pathway and other transcriptional factors.
Results: Hibiscus extract inhibited the adipocyte differentiation of 3T3-L1 preadipocytes induced by insulin, dexamethasone, and isobutylmethylxanthine (IBMX) in a dose-dependent manner. Hibiscus blocked the cytoplasmic lipid accumulation when administered at the onset of differentiation and 4 days after induction of differentiation. The inhibitory effect of hibiscus on adipogenic lipid accumulation of preadipocytes was significant (p < 0.01) between control cells and cells treated with hibiscus. Hibiscus extract significantly attenuated the expression of key adipogenic transcription factors, including CCAAT element binding protein (C/EBP)alpha and peroxisome proliferator-activated receptor (PPAR)gamma at protein levels.
Conclusion: These results suggest that hibiscus extract blocks adipogenesis, in part, by its suppression on the expression of adipogenic transcription factors, including C/EBPalpha and PPARgamma.
Synergism of plant-derived Polyphenols in Adipogenesis : perspectives and implications
Effect of Hibiscus sabdariffa extract on high fat diet–induced Obesity and liver damage in hamsters
Background: Obesity is a chronic metabolic disorder associated with an increase in adipogenesis and often accompanied with fatty liver disease.
Objective: In this study, we investigated the anti-obesity effects of Hibiscus sabdariffa water extract (HSE) in vivo.
Method: Eight-weeks-old male mice were divided into six groups (n=8 per group) and were fed either normal feed, a high fat diet (HFD), HFD supplemented with different concentrations of HSE, or HFD supplemented with anthocyanin. After 10 weeks of feeding, all the blood and livers were collected for further analysis.
Results: Mesocricetus auratus hamster fed with a high-fat diet developed symptoms of obesity, as determined from their body weight change and from their plasma lipid levels. Meanwhile, HSE treatment reduced fat
Therapeutic potential of Hibiscus sabdariffa: a review of the scientific evidence
Results: Results of the different articles suggested a possible therapeutic effect of H. sabdariffa extracts on oxidative stress, lipid profile, hypertension, and atherosclerosis thanks to its composition rich in phenolic compounds. Anthocyanins significantly decrease LDL oxidation, inhibit adipogenesis by regulating adipogenic signaling pathways and transcription factors, and modulate gene expression of certain microRNAs. No adverse events or side effects were reported.
Conclusions: Further more homogeneous, placebo-controlled studies in humans are needed to state that H. sabdariffa has therapeutic efficacy in humans.

Phenolic compounds and Flavonoids ameliorate bodyweight, blood glucose, and serum lipid profile. Since seabuckthorn (Hippophae rhamnoides L.) is known as a rich source of isoflavones and Flavonoids , we hypothesized that ethanolic extract of seabuckthorn leaves (SL) may have Anti-Obesity and hypoglycemic effects. To investigate the effect of ethanolic extract of SL, 32 C57BL/6J mice were randomly divided into 4 dietary groups, containing 8 mice in each group: normal diet group; high-fat diet (HD) control group; high-fat diet with SL extract, 500 mg/kg body weight (BW) (SL1) group; and high-fat diet with SL extract, 1000 mg/kg BW (SL2) group. After 13 weeks, it was observed that oral administration of SL extract significantly Reduced the energy intake; BW gain; epididymal fat pad weight; hepatic triglyceride, hepatic, and serum total cholesterol levels; and serum leptin levels in the SL groups compared to the HD group. However, differences in serum triglyceride and insulin levels in the SL groups were not significant in comparison to the HD group.
Results: Hyperoside in high concentrations significantly suppressed the Adipogenic process by Inhibiting the expression of transcription factors and Adipogenic genes and reducing lipid accumulation in Adipocytes (p<0.05). Low doses of hyperoside are able to Inhibit Adipogenesis , but higher doses are needed to Reduce Fat Accumulation in mature Adipocytes. In the case of maturing preadipocytes, 5 μM of hyperoside exerts its AntiAdipogenic effect at the early stages of Adipogenesis, whereas 10 μM of hyperoside acts at the later stages (p<0.05).
Conclusion: These results suggest that hyperoside has a beneficial effect on the prevention and treatment of Obesity.
Hypericum perforatum
In the present study, HpE not only showed hypolipidemic activity in normal rats but also normalised the lipid abnormalities induced by HFD or fructose feeding. Effects of HpE on lipid parameters are consonant with the earlier finding of Laggner et al. [26]. HpE also significantly Reduced the increased plasma glucose level in fructose or HFD-fed rats indicating improvement in insulin functions. Thus, reversal of fructose-induced insulin resistance appears to be the likely mechanism responsible for the observed effects of HpE on lipid parameters.
HpE showed significant Inhibition in Weight Gain induced by high-fat diet or fructose feeding. Serotonin is important neurotransmitter that controls increase in body Mass and is involved in the pathophysiology of Obesity as well as depression [27]. Serotonin release is increased upon intake of carbohydrates. Serotonin regulates the overconsumption of carbohydrate-rich foods. Serotonin has also been reported to decrease food intake in fructose-fed rats [28]. Hypericum perforatum increases the quantity of serotonin present within synaptosomes by Inhibiting synaptosomal uptake of serotonin [29]. This increased level of serotonin caused by HpE Reduces the food intake and Suppresses the appetite [30, 31]. Thus increased serotonergic transmission might be the connecting link between antidepressant- and AntiObesity -like activity of HpE.
Icaritin
Icaritin stimulates osteogenic Differentiation and Inhibits Adipogenesis of mesenchymal stem cells
This was one of the first studies demonstrating that the semisynthetic molecule Icaritin could promote osteogenic Differentiation and Inhibit their Adipogenesis of MSCs. These results demonstrate also that the pro-osteogenic effect of Icaritin is associated with regulation of GSK3β activity, and its Anti-Adipogenic effect is associated with blocking PPARγ signaling. The in vitro study indicates that Icaritin has the potential for further development into anti-osteoporosis drug.
Irvingia gabonensis
Results: The IGOB131 significantly Inhibited Adipogenesis in Adipocytes . The effect appears to be mediated through the down-regulated expression of Adipogenic transcription factors (PPAR gamma) [P less than 0.05] and Adipocyte -specific proteins (leptin) [P less than 0.05], and by up-regulated expression of adiponectin [P less than 0.05].
Conclusion: IGOB131 may play an important multifaceted role in the control of Adipogenesis and have further implications in in-vivo anti Obesity effects by targeting the PPAR gamma gene, a known contributory factor to Obesity in humans.
Ivy gourd (Coccinia grandis)
Ivy gourd (Coccinia grandis L. Voigt) root Suppresses Adipocyte Differentiation in 3T3-L1 cells
Our results have shown for the first time, that ivy gourd root possessed an Anti-Obesity property. It acted directly on Pre-Adipocytes by Inhibiting their Differentiation through down-regulation of at least the key Adipogenic transcription factor-PPARγ. The presence of possible Anti-Adipogenic agent in this plant might be relevant to its use to improve metabolic diseases induced by Obesity, in addition to having a blood sugar lowering effect. We are now attempting to identify its active component(s). Further study is also necessary to evaluate the Anti-Obesity effect of ivy gourd root in experimental animals.
Kaempferol
TAZ is required for the osteogenic and Anti-Adipogenic activities of kaempferol
KMP also enhanced the association of TAZ with PPARγ, thereby suppressing the gene transcription of PPARγ targets and resulting in diminished adipocyte differentiation. Interestingly, the regulatory effects of kaempferol on RUNX2 and PPARγ-mediated transcriptional activity were impaired in TAZ-null mouse embryonic fibroblasts but recovered by restoration of TAZ expression. Our results demonstrate that KMP fortifies TAZ activity, which enhances RUNX2-mediated osteoblast differentiation and suppresses PPARγ-stimulated adipocyte differentiation, indicating the potential of KMP as an effective therapeutic reagent for controlling bone loss and adiposity through TAZ activation.
Anti-Adipogenic effect of kaempferol, a component of Polygonati rhizoma
Kaempferol is a natural flavonoid widely found in fruits, vegetables, and tea. Kaempferol possesses beneficial biological properties such as anti-inflammatory and antioxidant activities. Positive energy balance during obesity correlates with a pro-inflammatory chronic state. In this context, we hypothesized that kaempferol might promote anti-obesity effects by modulating adipogenesis and lipolytic pathways. Adipocyte viability at 24, 48, and 72 h was measured by an ATP-based assay. Pre-adipocytes (day 0) or mature adipocytes (day 12) were treated with 60 μM kaempferol until day 21 to evaluate its potential anti-adipogenic and lipolytic effect, respectively.
Additionally, the mRNA expression of adipogenesis genes decreased on treatment with kaempferol. The role of kaempferol at the genome-wide level was further assessed by a microarray approach. Our analysis indicated that kaempferol decreased the expression of adipogenic transcription factors (Pparγ, Cebpβ, Srebp1, Rxrβ, Lxrβ, Rorα) and genes involved in triglyceride biosynthesis (Gpd1, Agpat2, Dgat2), while increasing lipolysis-related genes, such as Tnfα, Lsr, and Cel. Finally, co-transfection assays using luciferase reporter gene and reverse transcription-polymerase chain reaction (RT-PCR) analysis using peroxisome proliferator-activated receptor-γ (PPARγ) target genes indicated that kaempferol significantly repressed rosiglitazone-induced PPARγ transcriptional activity.
Overall, our data suggests that kaempferol, a major component of RPF, may be beneficial in obesity, by reducing adipogenesis and balancing lipid homeostasis partly through the down-regulation of PPARγ.
In this study, the anti-adipogenetic activity of 300 plant extracts was investigated using an Oil Red O staining assay in a 3T3-L1 cell line. Our results indicate that three plants, including the stem and leaf of Physalis angulata, the whole grass of Solidago virgaurea, and the root of Dioscorea nipponica, produced over 90% inhibition of adipogenesis.
Kaempferol-3-O-rutinoside, which demonstrated a 48.2% inhibitory effect on adipogenesis without cytotoxicity, was isolated from the butanol layer of a water extract of S. virgaurea guided by the anti-adipogenesis assay in 3T3-L1. PPAR-γ and C/EBPα expression levels were determined using western blot, and our results indicate that kaempferol-3-O-rutinoside has a strong anti-adipogenic effect in 3T3-L1 cells through the suppression of increases in PPAR-γ and C/EBPα expression.
This study was designed to analyze the anti-adipogenic effect of fifteen phenolic compounds from various chemical groups in 3T3-L1 pre-adipocytes. Cells were treated with 25 μM, 10 μM or 1 μM of apigenin, luteolin, catechin, epicatechin, epigallocatechin, genistein, daizein, naringenin, hesperidin, quercetin, kaempferol, resveratrol, vanillic acid, piceatannol and pterostilbene for 8 days. At 25 μM lipid accumulation was reduced by all the compounds, with the exception of catechin, epicatechin and epigallocatechin.
Results: Apigenin did not show an anti-adipogenic action. Pre-adipocytes treated with hesperidin and kaempferol showed reduced TG content at the three experimental doses. Apigenin did not modify the expression of the main adipogenic genes (c/ebpβ, c/ebpα, pparγ and srebp1c), hesperidin inhibited genes involved in the three phases of adipogenesis (c/ebpβ, srebp1c and perilipin) and kaempferol reduced c/ebpβ. In mature adipocytes, the three polyphenols reduced TG accumulation at the dose of 25 µM, but not at lower doses. All compounds increased mRNA levels of atgl. Apigenin and hesperidin decreased fasn expression. The present study shows the anti-adipogenic effect and delipidating effects of apigenin, hesperidin and kaempferol in human adipocytes derived from hMSCs. While hesperidin blocks all the stages of adipogenesis, kaempferol only inhibits the early stage. Regarding mature adipocytes, the three compounds reduce TG accumulation by activating, at least in part, lipolysis, and in the case of hesperidin and apigenin, also by reducing lipogenesis.
Conclusions: The present study shows for the first time the anti-adipogenic effect and delipidating effect of apigenin, hesperidin and kaempferol in human adipocytes derived from MSCs for the first time.
Keywords: Adipocytes; Apigenin; Hesperidin; Human mesenchymal stem cells; Kaempferol; Obesity.
Euonymus alatus as a folk medicine in China has been clinically used to treat type 2 diabetes for many years, and also exerts beneficial effects on hyperglycemia of diabetic animals. Our previous studies have isolated kaempferol and quercetin from the extract of E. alatus. In the present study, we investigated the possible mechanism of antidiabetic activity of these compounds. Kaempferol and quercetin could significantly improve insulin-stimulated glucose uptake in mature 3T3-L1 adipocytes. In addition, further experiments showed that kaempferol and quercetin served as weak partial agonists in the peroxisome proliferator-agonist receptor gamma (PPARgamma) reporter gene assay. Kaempferol and quercetin could not induce differentiation of 3T3-L1 preadipocytes as traditional PPARgamma agonist.

In mature Adipocytes, GEFr was found to significantly activate AMP-activated protein kinase (AMPK) by activating liver kinase B1 (LKB1) and stimulating intracellular reactive oxygen species generation. The mRNA levels of genes involved in lipid catabolism were up-regulated. Also, GEFr increased Lipolysis in a dose-dependent manner.
Taken together, these results suggest that GEFr has potential for use in therapies designed to improve Obesity.

Adenophora triphylla var. japonica (Campanulaceae) is known to have Anti‐Inflammatory and anti‐tussive effects. Dysfunction of Adipocytes and Adipose Tissue in Obesity is related to various inflammatory cytokines or adipokines. In this study, we investigated whether lupenone isolated from A. triphylla var. japonica extract Inhibits Adipocyte Differentiation and expression of Adipogenic marker genes in 3T3‐L1 preadipocytes . We demonstrated that lupenone resulted in a significant reduction in lipid accumulation and expression of Adipogenic marker genes in a dose‐dependent manner.
In addition, lupenone decreased the transcriptional activity of peroxisome proliferator‐activated receptor γ (PPARγ ) induced by troglitazone, and we also demonstrated that lupenone suppressed the PPARγ and CCAAT‐enhancer‐binding protein α (C/EBPα ) protein levels. These findings demonstrated that lupenone isolated from A. triphylla var. japonica extract effectively Inhibited Adipocyte Differentiation through downregulation of related transcription factor, particularly the PPARγ gene.
Inhibitory effects of lupeol on 3T3-L1 Preadipocyte Differentiation
In the Differentiation of a 3T3-L1 Preadipocyte to a mature Adipocyte , intensive triglyceride (TG) synthesis and intracellular accumulation of lipid droplets are induced. Lupeol, a lupane triterpene, markedly blocked TG synthesis and the accumulation of lipid droplets in 3T3-L1 cells stimulated with Differentiation inducers.
The analysis of gene expressions by quantitative real-time RT-PCR demonstrated that lupeol markedly Inhibited Adipogenic transcription factors, Adipogenic enzymes, and adipocytokines in mature 3T3-L1 cells. These findings suggested that lupeol strongly Inhibited 3T3-L1 cell Differentiation into mature Adipocytes in vitro.

LFE stimulated fatty acid oxidation in an AMPK-dependent manner. In high-fat diet (HFD)-induced obese mice (n = 8/group), oral administration of LFE at 30, 100, and 300 mg/kg/day decreased total body Weight Gain significantly in all doses tested. No difference in food intake was observed between vehicle- and LFE-treated HFD mice. The weight of white Adipose Tissue s including abdominal subcutaneous, epididymal, and perirenal Adipose Tissue was Reduced markedly in LFE-treated HFD mice in a dose-dependent manner.
Treatment of LFE also greatly improved serum levels of Obesity-related biomarkers such as glucose, triglycerides, and adipocytokines leptin, adiponectin, and resistin. All together, these results showed Anti-Obesity effects of LFE on Adipogenesis and lipid metabolism in vitro and in vivo and raised a possibility of developing LFE as Anti-Obesity therapeutics.
Key findings: Magnolol dose-dependently enhanced Adipocyte Differentiation in 3T3-L1 cells and C3H10T1/2 cells. In the early stage of Adipogenesis , magnolol induced gene expression of C/EBPδ, C/EBPα and PPARγ 2 and during Adipocyte Differentiation , it also induced the expression of PPARγ target genes such as aP2, LPL and adiponectin. In addition, magnolol it also increase expression of PAPRγ target gene such as C/EBPα and aP2 at mRNA and aP2 protein level in mature Adipocytes . In PPARγ ligand binding assays, magnolol exhibited binding affinity to PPARγ but its activity was weaker than rosiglitazone. At the same time, magnolol-induced Adipogenesis was Inhibited by co-treatment of GW9662 both 3T3-L1 cells and C3H10T1/2 cells. In mature 3T3-L1 Adipocytes , magnolol increased basal and insulin-stimulated glucose uptake accompanied by the up-regulation of mRNA and protein level of Glut4.
Significance: Our results suggest that magnolol could improve insulin sensitivity through the activation of PPARγ as a ligand.
Kudinoside-D (Ilex kudingcha)
The Adipocytes were treated with various concentrations of kudinoside D (0 to 40 μM) during Differentiation . The image-based Oil Red O staining analyses revealed that KD-D, dose dependently Reduced cytoplasmic lipid droplet in 3T3-L1 Adipocytes with the IC50 is 59.49 μM. Meanwhile, major Adipogenic transcription factor peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer binding protein-α (C/EBPα) and sterol regulatory element-binding protein 1c (SREBP-1c) were significantly repressed as well as their target genes. The phosphorylation of AMP-activated protein kinase (AMPK) and its downstream target phosphorylated-acetyl CoA carboxylase (ACC) expression were also increased.
In addition, the Inhibitory effects of KD-D on the expressions of PPARγ and C/EBPα were weakened when cells were cotreated with AMPK Inhibit or Compound C. These results indicated KD-D exerts Anti-Adipogenic effects through modulation of Adipogenic transcription factors via AMPK signaling pathway. And the current findings demonstrated that KD-D was a potential therapeutic candidate for alleviating Obesity and hyperlipidemia.
Mangifera indica
Expansion of Adipose Tissue in Obesity is associated with dysregulation of adipokines, which can lead to long-term metabolic disorders. Gallotannin derivatives from mango possess anti-inflammatory activities, but their potential role in lipid metabolism is not well investigated. In this study, 3T3-L1 preadipocytes were differentiated into Adipocytes and treated with mango Polyphenols (MG), or pyrogallol (PG) for 6 days. The Anti-Adipogenic activity of PG was demonstrated by Reduced number of lipid droplets and expressions of Adipogenic markers, such as peroxisome proliferator-activated receptor γ (PPARγ ) and CCAAT/enhancer binding protein α (C/EBPα).
In mature Adipocytes, PG promoted Adipocyte browning and increased the expressions of uncoupling protein 1 (UCP1) and sirtuin 1 (Sirt1). Knockdown of AMP-activated protein kinase (AMPK) α1 with siRNA partially abolished the effect of PG on Adipogenesis. Results indicate that gallotannin derivatives modulate lipid metabolism, at least in part, though the AMPK pathway, and possess potential to Prevent Obesity-related conditions.
We aimed to examine the anti‐adipogenic effects of mangiferin (xanthone glucoside) and its related mechanism. Human mesenchymal stem cells (hMSCs) were cultured in Dulbecco’s modified Eagle medium, after 60% confluence adipocyte differentiation was induced. Differentiation‐induced hMSCs were cultured in the presence and absence of 10, 20 and 40 μmol of mangiferin from day 0 to day 10. Adipocyte differentiation and lipid accumulation were significantly decreased in 40 μmol mangiferin‐treated groups when compared with the reference drugs (quercetin‐ and orlistat‐treated groups).
Using quantitative reverse transcription polymerase chain reaction, we studied the mRNA expression levels of resistin, adipocyte fatty acid‐binding protein 2 (aP2), lipoprotein lipase (LPL), peroxisome proliferator‐activated receptor (PPAR‐γ) and tumor necrosis factor‐α, in hMSCs undergoing adipocyte differentiation; treatment with mangiferin attenuated the expression of those adipogenic genes and decreased adipocyte differentiation. Mangiferin significantly inhibited hMSCs to preadipocyte differentiation within the first 2 days of treatment, indicating that the anti‐adipogenic effects of mangiferin are achieved through the inhibition of differentiation and maturation.
Natural bioactive compounds may be used in obese patients because of their ability to impact on various key mechanisms involved in the complex pathophysiological mechanisms of such condition. The aim of this study was to investigate the effect of a Mangifera indica L. leaf extract (MLE) on adipogenic differentiation of murine preadipocyte cells. 3T3-L1 cells were treated during their differentiation with various concentrations of (Mangifera indica L.) leaves extract (MLE) (750, 380, 150, 75 and 35 μg) in order to assess their lipid content, adiponectin production, expression profile of genes involved in lipid metabolism, oxidative stress and inflammation.

Leaves of Ilex paraguariensis are used to prepare a tea known as maté which is a common beverage in several South American countries. The ethanol extract was fractionated to identify the compounds responsible for the Anti-Adipogenic activity in 3T3-L1 cells . Extracts of both fresh and dried maté leaves were subjected to column chromatography using molecular permeation to obtain the saponin (20 % yields) and the Polyphenol extracts (40 % yields) from the fresh and dried leaves. The phenolic content was determined using high-performance liquid chromatography analysis and the Folin-Ciocalteau method. Also, maté extracts (50 μg/ml to 1,000 μg/ml) did not display citotoxicity using MTT.
The Polyphenol extract from the dried leaves was the most effective (50 μg/ml) in the Inhibition of triglyceride accumulation in 3T3-L1 Adipocytes, and rutin (100 μg/ml) likely accounted for a large portion of this activity. Additionally, maté extracts had a modulatory effect on the expression of genes related to the Adipogenesis as PPARγ 2, leptin, TNF-α and C/EBPα.
Melissa officinalis
Results: Treatment of cells with Ob-X inhibited lipid accumulation and adipocyte-specific gene expression caused by troglitazone or monocyte differentiation-inducing (MDI) mix. Ob-X reduced mRNA levels of angiogenic factors (vascular endothelial growth factor-A, -B, -C, -D, and fibroblast growth factor-2) and matrix metalloproteinases (MMPs; MMP-2 and MMP-9), whereas it increased mRNA levels of angiogenic inhibitors [(thrombospondin-1, tissue inhibitor of metalloproteinase-1 (TIMP-1), and TIMP-2)] in differentiated cells. MMP-2 and MMP-9 activities were also decreased in Ob-X-treated cells.
Discussion and conclusion: These results suggest that the anti-angiogenic herbal composition Ob-X inhibits differentiation of preadipocytes into adipocytes. These events may be mediated by changes in the expression of genes involved in lipogenesis, angiogenesis, and the MMP system. Thus, by reducing adipogenesis, anti-angiogenic Ob-X provides a possible therapeutic approach for the prevention and treatment of human obesity and its related disorders.
Results: ALS-L1023 inhibited angiogenesis in a dose-dependent manner in the HUVEC tube formation assay in vitro. Treatment of cells with ALS-L1023 inhibited lipid accumulation and adipocyte-specific gene expression caused by troglitazone or MDI differentiation mix. ALS-L1023 reduced mRNA expression of angiogenic factors (VEGF-A and FGF-2) and MMPs (MMP-2 and MMP-9) in differentiated cells. In contrast, mRNA levels of angiogenic inhibitors (TSP-1, TIMP-1, and TIMP-2) increased. Protease activity, as measured by zymography, showed that activity of MMP-2 and MMP-9 decreased in ALS-L1023-treated cells. ALS-L1023 also inhibited MMP-2 and MMP-9 reporter gene expression in the presence of the MMP inducer phorbol 12-myristate 13-acetate. An in vivo study showed that ALS-L1023 not only decreased adipose tissue mass and adipocyte size, but also reduced mRNA levels of adipose tissue angiogenic factors and MMPs in HFD-fed obese mice.
Conclusions: These results suggest that the anti-angiogenic herbal extract ALS-L1023 suppresses adipogenesis and adipocyte hypertrophy, and this effect may be mediated by inhibiting angiogenesis and MMP activities. Thus, by curbing adipogenesis, anti-angiogenic ALS-L1023 yields a possible therapeutic choice for the prevention and treatment of human obesity and its associated conditions.
Methyl cinnamate (Zanthoxylum armatum)
The aim of this study was to investigate the Inhibitory effect of methyl cinnamate on Adipogenesis in 3T3-L1 preadipocytes. Methyl cinnamate markedly suppressed triglyceride accumulation associated with down-regulation of Adipogenic transcription factor expression, including sterol regulatory element binding protein-1 (SREBP-1), peroxisome proliferator-activated receptor γ (PPARγ ), and CCAAT/enhancer-binding protein α (C/EBPα).
Additionally, methyl cinnamate-Inhibited PPARγ activity and Adipocyte Differentiation were partially reversed by the PPARγ agonist troglitazone. Furthermore, methyl cinnamate stimulated Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) and phospho-AMP-activated protein kinase (AMPK) expression during Adipogenesis. This study first revealed methyl cinnamate has AntiAdipogenic activity through mechanisms mediated, in part, by the CaMKK2–AMPK signaling pathway in 3T3-L1 cells.
Mollugin (Rubia cordifolia)
Effect of mollugin on apoptosis and Adipogenesis of 3T3‐L1 preadipocytes
The effect of mollugin, isolated from the roots of Rubia cordifolia L., on cell viability, apoptosis and Adipogenesis in 3T3‐L1 preadipocytes was investigated. The Inhibitory effect of mollugin (40–60 µm) on cell viability was more significant in differentiated Adipocytes than in 3T3‐L1 preadipocytes . In 3T3‐L1 cells, the cytotoxicity of mollugin was accompanied by apoptotic events including mitochondrial membrane potential (Δψm) loss and activation of caspase‐9, ‐3 and ‐7, leading to PARP degradation. Although the presence of 20 µm mollugin during induced adipocytic Differentiation of 3T3‐L1 cells for 6 days failed to affect the cell viability, it could almost completely abrogate the Differentiation‐associated morphology change and intracellular lipid accumulation.
A similar level of Inhibition was observed, when 20 µm mollugin was present during the early stage (D0–D2) of the Differentiation period. In addition, the expression of C/EBPα, PPARγ 1 and PPARγ 2 was significantly down‐regulated. The presence of 20 µm mollugin during either middle stage (D2–D4) or late stage (D4–D6) of the Differentiation period, however, caused the Inhibition to a lesser extent. These results indicated that mollugin at high concentrations (40–60 µm) exerted cytotoxicity via inducing apoptosis, whereas mollugin at a low concentration (20 µm) suppressed adipocytic Differentiation without exerting cytotoxicity in 3T3‐L1 preadipocytes.
Momordica charantia
Results: Preadipocytes treated with varying concentrations of BMJ during Differentiation demonstrated significant reduction in lipid content with a concomitant reduction in mRNA expression of Adipocyte transcription factors such as, peroxisome proliferator-associated receptor γ (PPARγ ) and sterol regulatory element-binding protein 1c (SREBP-1c) and adipocytokine, resistin. Similarly, Adipocytes treated with BMJ for 48 h demonstrated Reduced lipid content, perilipin mRNA expression, and increased Lipolysis as measured by the release of glycerol.
Conclusion: Our data suggests that BMJ is a potent Inhibit or of lipogenesis and stimulator of Lipolysis activity in human Adipocytes. BMJ may therefore prove to be an effective complementary or alternative therapy to Reduce Adipogenesis in humans.
Moringa oleifera
The iso-quercetin and MLE showed anti-proliferative activity at the concentration of 4.95 µg/mL and 280.5 µg/mL respectively. The expression of adipogenesis related genes were downregulated with increased concentration of MLE treatment and caused the decreased accumulation of triglyceride content. Furthermore, MLE induced apoptosis in adipocyte cells were confirmed by TUNEL, Hoechst and PI staining. The apoptosis pathway related pathway genes such as BAX, BCL2 were also studied. Upon MLE treatment, BAX, a pro-apoptotic protein was up-regulated and BCL2, an anti-apoptotic protein was down-regulated and showed increased activity of caspase 3 indicating that apoptosis of adipocyte cells.
In recent years, obesity has become a key factor affecting human health. Moringa oleifera Lam. is a perennial tropical deciduous tree, which is widely used in human medicine due to its nutritional and unique medicinal value. It has a cholesterol-lowering effect, but its mechanism of action is unclear. In this study, we elucidated the inhibitory effect of M. oleifera leaf petroleum ether extract (MOPEE) on lipid accumulation by in vitro and in vivo experiments, and we described its mechanism of action. MOPEE suppressed adipogenesis in 3T3-L1 adipocytes in a dose-dependent manner and had no effect on cell viability at doses up to 400 μg/ml. Furthermore, MOPEE (400 μg/ml) significantly downregulated the expression of adipogenesis-associated proteins [peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer-binding proteins α and β (C/EBPα and C/EBPβ), and fatty acid synthase (FAS)] and upregulated the expression of a lipolysis-associated protein [hormone-sensitive lipase (HSL)] in 3T3-L1 adipocytes.
Moringa oleifera and Obesity : A review
Moringaoleifera Lam [Moringaceae] is a highly valued plant, distributed in many countries of the tropics and subtropics. It has an impressive range of medicinal uses with high nutritional value. Different parts of this plant contain a profile of important minerals, and are a good source of protein, vitamins, β-carotene, amino acids and various phenolics. In addition to its compelling water purifying powers and high nutritional value, M. oleifera is very important for its medicinal value. Various parts of this plant such as the leaves, roots, seed, bark, fruit, flowers and immature pods act as cardiac and circulatory stimulants, possess antitumor, antipyretic, antiepileptic, anti-inflammatory, antiulcer, antispasmodic, diuretic, antihypertensive, cholesterol lowering, antioxidant, antidiabetic, hepatoprotective, antibacterial and antifungal activities, and are being employed for the treatment of different ailments in the indigenous system of medicine.
Morus alba
Mulberry leaf, an important traditional Chinese medicine, possesses many biological activities, including effects of anti-obesity. However, which constituents of mulberry leaf are responsible for its anti-adipogenic action is unclear. This study primarily investigated the chemical constituents from mulberry leaf and their bioactivity on the proliferation and differentiation of 3T3-L1 preadipocytes.
A new flavane derivative, (2S)-4′-hydroxy-7-methoxy-8-prenylflavan (1), together with twelve known compounds including three flavanes (2-4), three chalcones (5-7), two flavones (8-9), two benzofurans (10-11) and two coumarin (12-13) was isolated from mulberry leaf. The structure of the new compound was elucidated by various spectroscopic methods including UV, HR-ESI-MS, (1)H and (13)C NMR and CD. The results of activity screening showed that compound 2, 6 and 7 inhibited the proliferation and differentiation of 3T3-L1 preadipocytes.
Results: Treatment of cells with Ob-X inhibited lipid accumulation and adipocyte-specific gene expression caused by troglitazone or monocyte differentiation-inducing (MDI) mix. Ob-X reduced mRNA levels of angiogenic factors (vascular endothelial growth factor-A, -B, -C, -D, and fibroblast growth factor-2) and matrix metalloproteinases (MMPs; MMP-2 and MMP-9), whereas it increased mRNA levels of angiogenic inhibitors [(thrombospondin-1, tissue inhibitor of metalloproteinase-1 (TIMP-1), and TIMP-2)] in differentiated cells. MMP-2 and MMP-9 activities were also decreased in Ob-X-treated cells.
Discussion and conclusion: These results suggest that the anti-angiogenic herbal composition Ob-X inhibits differentiation of preadipocytes into adipocytes. These events may be mediated by changes in the expression of genes involved in lipogenesis, angiogenesis, and the MMP system. Thus, by reducing adipogenesis, anti-angiogenic Ob-X provides a possible therapeutic approach for the prevention and treatment of human obesity and its related disorders.
The antioxidant and anti-adipogenic activities of a mixture of Nelumbo nucifera L., Morus alba L., and Raphanus sativus were investigated and their anti-obesity activities were established in vitro and in vivo. Among the 26 different mixtures of extraction solvent and mixture ratios, ethanol extract mixture no. 1 (EM01) showed the highest antioxidant (α,α-Diphenyl-β-picrylhydrazyl, total phenolic contents) and anti-adipogenic (Oil-Red O staining) activities. EM01 inhibited lipid accumulation in 3T3-L1 adipocytes compared to quercetin-3-O-glucuronide.
Furthermore, body, liver, and adipose tissue weights decreased in the high-fat diet (HFD)-EM01 group compared to in the high-fat diet control group (HFD-CTL). EM01 lowered blood glucose levels elevated by the HFD. Lipid profiles were improved following EM01 treatment. Serum adiponectin significantly increased, while leptin, insulin growth factor-1, non-esterified fatty acid, and glucose significantly decreased in the HFD-EM01 group. Adipogenesis and lipogenesis-related genes were suppressed, while fat oxidation-related genes increased following EM01 administration. Thus, EM01 may be a natural anti-obesity agent.
Anti-oxidant and anti-obese effects of mulberry (Morus alba L.) leaf extract in 3T3-L1 cells
Fruits of mulberry (Morus alba) have been widely used for therapeutic purposes in Asian countries for centuries. Treatment of 3T3-L1 cells with ethanolic extracts of M. alba decreased adipocyte differentiation at 100 microg/mL by 18.6%. Treatment suppressed mRNA levels of PPARgamma and C/EBPalpha expression in 3T3-L1 cells. However, the extract did not change free glycerol release from mature adipocytes. Thus, M. alba inhibited lipid accumulation by regulating transcription factors in 3T3-L1 adipocytes without a lipolytic effect. Among the soluble- fractions, the ethyl acetate-soluble fraction had the highest antiadipogenic effects on 3T3-L1 cells.
Anti-Adipogenic effect of mulberry leaf ethanol extract in 3T3-L1 Adipocytes
Results: Results showed that MLEE treatments at 10, 25, 50, and 100 µg/ml had no effect on cell morphology and viability. Without evident toxicity, all MLEE treated cells had lower fat accumulation compared with control as shown by lower absorbances of Oil Red O stain. MLEE at 50 and 100 µg/ml significantly reduced protein levels of PPARγ, PGC-1α, FAS, and adiponectin in differentiated adipocytes. Furthermore, protein level of C/EBPα was significantly decreased by the treatment of 100 µg/ml MLEE.
Conclusion: These results demonstrate that MLEE treatment has an anti-adipogenic effect in differentiated adipocytes without toxicity, suggesting its potential as an anti-obesity therapeutic.

Structures of these compounds were identified using various spectral data. Each of these compounds also Inhibited Adipogenesis in 3T3-L1 cells. In particular, compound 3 was a more potent Inhibit or of triglyceride accumulation than the positive control berberine. Gene expression studies revealed that treatment of 3T3-L1 cells with 3 lowers the expression levels of CCAAT/enhancer-binding protein α and peroxisome proliferator activator γ2 during Adipogenesis without affecting cell viability.
Treatment of 3T3-L1 cells with 3 Reduced the expression levels of mRNAs encoding sterol regulatory element-binding protein 1c and several lipogenic enzymes, including fatty acid synthase and stearoyl CoA desaturase-1. These results indicate that the methanol extract and compounds isolated from the dried branches and leaves of murta exert their Anti-Obesity effects through the Inhibition of Adipogenesis.

This study evaluated potential AntiDiabetic and AntiObesity properties in vitro of selected medicinal plants. The hot water (WE) and ethanol extracts (EE) of sweet gale (Myrica gale L.), roseroot (Rhodiola rosea L.), sheep sorrel (Rumex acetosa L.), stinging nettles (Utrica dioica L.) and dandelion (Taraxacum officinale L.) were tested for total antioxidant capacity using ferric reducing antioxidant power (FRAP) and DPPH• scavenging capacity assays, followed by α-amylase, α-glucosidase and formation of advanced glycation end products (AGE) Inhibition assays in vitro.
This study aimed to assess the anti-Diabetic activity in vitro of plant resources used in traditional medicine in north Newfoundland region of Canada. The extracts prepared from sweet gale (Myrica gale L.), roseroot (Rhodiola rosea L.), sheep sorrel (Rumex acetosa L.), stinging nettles (Utrica dioica L.), and dandelion (Taraxacum officinale L.) were used to compare their antioxidant and AntiDiabetic properties in vitro. The study was extended further to explore and evaluate the AntiAdipogenic ability of selected plant extracts in vitro using 3T3-L1 preadipocytes.
These studies have largely focused on the effects of naringenin on the liver. Our biochemical studies clearly demonstrate that naringenin Inhibits Adipogenesis and impairs mature fat cell function. Naringenin specifically Inhibited Adipogenesis in a dose-dependent fashion as judged by examining lipid accumulation and induction of Adipocyte marker protein expression. In mature 3T3-L1 Adipocytes , naringenin Reduced the ability of insulin to induce IRS-1 tyrosine phosphorylation and substantially Inhibited insulin-stimulated glucose uptake in a dose-dependent manner and over a time frame of 1.5 to 24 hours.
Exposure to naringenin also Inhibited adiponectin protein expression in mature murine and human Adipocytes. Our studies have revealed that naringenin may have a negative impact on Adipocyte-related diseases by limiting Differentiation of preadipocytes, by significantly inducing insulin resistance, and by decreasing adiponectin expression in mature fat cells.

Key findings: We demonstrated that the combined treatment of Differentiation reagents with nobiletin or tangeretin differentiated 3T3-L1 preadipocytes into Adipocytes possessing less intracellular triglyceride as compared to vehicle-treated differentiated 3T3-L1 Adipocytes . Both Flavonoids increased the secretion of an insulin-sensitizing factor, adiponectin, but concomitantly decreased the secretion of an insulin-resistance factor, MCP-1, in 3T3-L1 Adipocytes . Furthermore, nobiletin was found to decrease the secretion of resistin, which serves as an insulin-resistance factor. In mature 3T3-L1 Adipocytes , nobiletin induced apoptosis; tangeretin, in contrast, did not induce apoptosis, but suppressed further triglyceride accumulation.
Significance: Our results suggest that nobiletin and tangeretin are promising therapeutic candidates for the prevention and treatment of insulin resistance by modulating the adipocytokine secretion balance. We also demonstrated the different effects of nobiletin and tangeretin on mature Adipocytes.
Nonivamide (chilli pepper)
Red pepper and its major pungent principle, capsaicin (CAP), have been shown to be effective anti‐Obesity agents by reducing energy intake, enhancing energy metabolism, decreasing serum triacylglycerol content, and Inhibiting Adipogenesis via activation of the transient receptor potential cation channel subfamily V member 1 (TRPV1). However, the binding of CAP to the TRPV1 receptor is also responsible for its pungent sensation, strongly limiting its dietary intake. Here, the effects of a less pungent structural CAP‐analog, nonivamide, on Adipogenesis and underlying mechanisms in 3T3‐L1 cells were studied.
Nonivamide was found to Reduce mean lipid accumulation, a marker of Adipogenesis, to a similar extent as CAP, up to 10.4% (P < 0.001). Blockage of the TRPV1 receptor with the specific Inhibit or trans‐tert‐butylcyclohexanol revealed that the anti‐Adipogenic activity of nonivamide depends, as with CAP, on TRPV1 receptor activation. In addition, in cells treated with nonivamide during Adipogenesis, protein levels of the pro‐Adipogenic transcription factor peroxisome‐proliferator activated receptor γ (PPARγ ) decreased. Results from miRNA microarrays and digital droplet PCR analysis demonstrated an increase in the expression of the miRNA mmu‐let‐7d‐5p, which has been associated with decreased PPARγ levels.
nuciferine (Lotus Leaf)
Results: Ten μg/ml LLAE significantly increased the luciferase activities in 3T3-L1 cells and the stimulatory action reached 2.51 folds of controls when LLAE was 1000 μg/ml (P < 0.01). After treating 3T3-L1 cells with 100 μg/ml LLAE, the stimulatory role peaked at 32 h where it was 2.58 folds of controls (P < 0.01). Besides, 100 μg/ml LLAE significantly increased PPARγ2 mRNA levels in human adipocytes to 1.91 folds of controls (P < 0.01). In HFD-induced obese rats, administration with both low and high dose LLAE notably reduced visceral fat mass by 45.5 and 58.4%, respectively, and significantly decreased fasting serum insulin levels (P < 0.05). The high dose LLAE also significantly decreased homeostasis model assessment of insulin resistance in obese rats (P < 0.05). Furthermore, the mRNA levels of PPARγ2 and GLUT4 in VAT of obese rats were significantly increased when compared with control rats, and were notably suppressed by LLAE intervention for 6 weeks (P < 0.05).
Conclusion: LLAE significantly reduces visceral fat mass and ameliorates insulin resistance in HFD-induced obese rats. These beneficial effects of LLAE may associate with its role in stimulating PPARγ2 expression in preadipocytes and subcutaneous adipocytes and suppressing PPARγ2 and GLUT4 expression in VAT.
Oleanolic Acid (olea europaea l.leaves)
Oleanolic acid Reduce s markers of Differentiation in 3T3-L1 Adipocytes
Nontoxic oleanolic acid, at 25 μmol/L or less, dose-dependently attenuated lipid accumulation in differentiated Adipocytes as evidenced by Oil Red O staining. Western blot analysis showed that the induction of PPARγ and C/EBPα was markedly attenuated in differentiated and oleanolic acid-treated Adipocytes at their transcriptional messenger RNA levels. Furthermore, this study examined whether oleanolic acid dampened the induction of visfatin, a proinflammatory and visceral fat-specific adipokine expressed in Adipocytes. Visfatin expression was Inhibited in differentiated Adipocytes exposed to a PPARγ Inhibit or GW9662.
In addition, the visfatin production was significantly repressed in 25 μmol/L oleanolic acid-treated Adipocytes, possibly through blocking PPARγ activation. These results demonstrate that oleanolic acid may be a promising agent to disturb Adipocyte Differentiation and suppress Obesity-associated Inflammation.
Oligonol (Lychee fruit)
Polyphenols have recently become an important focus of study in Obesity research. Oligonol is an oligomerized Polyphenol , typically comprised of catechin-type Polyphenols from a variety of fruits, which has been found to exhibit better bioavailability and bioreactivity than natural Polyphenol compounds. Here, we demonstrated that Oligonol Inhibits 3T3-L1 Adipocyte Differentiation by reducing Adipogenic gene expression. During Adipogenesis, Oligonol downregulated the mRNA levels of peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer binding proteins α (C/EBPα), and δ (C/EBPδ) in a dose-dependent manner and the expression of genes involved in lipid biosynthesis.
The AntiAdipogenic effect of Oligonol appears to originate from its ability to Inhibit the Akt and mammalian target of rapamycin (mTOR) signaling pathway by diminishing the phosphorylation of ribosomal protein S6 kinase (p70S6K), a downstream target of mTOR and forkhead box protein O1 (Foxo1).
These results suggest that Oligonol may be a potent regulator of Obesity by repressing major Adipogenic genes through Inhibition of the Akt signaling pathway, which induces the Inhibition of lipid accumulation, ultimately Inhibiting Adipogenesis.
Orientin (Commelina communis)
Vitexin, orientin and other Flavonoids from Spirodela polyrhiza Inhibit Adipogenesis in 3T3‐L1 cells
To investigate the Adipogenesis Inhibitory effect on lipid accumulation, 3T3‐L1 cells were treated with fractions and isolated Flavonoids of Spirodela polyrhiza. An ethanol extract of S. polyrhiza was fractionated into three fractions. The butanol soluble fraction (SPB) exhibited potent AntiAdipogenesis activity and decreased C/EBPα and PPARγ protein expression level in 3T3‐L1 cells without significant cytotoxicity. The Flavonoids were isolated from SPB and their chemical structures were identified as chrysoeriol (1), apigenin (2), luteolin (3), vitexin (4), cosmosin (5), orientin (6) and luteolin‐7‐O‐β‐d‐glucoside (7) by spectroscopic analysis.
Studies on the Adipogenesis and intracellular triglyceride accumulation Inhibitory effect showed that compounds 4 and 6 had the highest activity and decreased C/EBPα and PPARγ protein expression level in 3T3‐L1 cells. These results suggest that the Flavonoids isolated from SPB, especially compounds 4 and 6, contribute to the Inhibitory activity of S. polyrhiza in 3T3‐L1 cells.
Oroxylin A (Oroxylum indicum)
Phloretin (Manchurian apricot)
Phloretin enhances Adipocyte Differentiation and adiponectin expression in 3T3-L1 cells
Adipocyte dysfunction is strongly associated with the development of cardiovascular risk factors and Diabetes. It is accepted that the regulation of Adipogenesis or adipokines expression, notably adiponectin, is able to Prevent these disorders. In this report, we show that phloretin, a dietary flavonoid, enhances 3T3-L1 Adipocyte Differentiation as evidenced by increased triglyceride accumulation and GPDH activity. At a molecular level, mRNA expression levels of both PPARγ and C/EBPα, the master Adipogenic transcription factors, are markedly increased by phloretin. Moreover, mRNA levels of PPARγ target genes such as LPL, aP2, CD36 and LXRα are up-regulated by phloretin.
We also show that phloretin enhances the expression and secretion of adiponectin. Co-transfection studies suggest the induction of PPARγ transcriptional activity as a possible mechanism underlying the phloretin-mediated effects. Taken together, these results suggest that phloretin may be beneficial for reducing insulin resistance through its potency to regulate Adipocyte Differentiation and function.
Methods and results: Differentiated 3T3‐L1 cells were treated with PT or PZ. Subsequently, transcription factors of adipogenesis and lipolysis proteins were measured. In addition, RAW 264.7 macrophages treated with PT or PZ were cultured in differentiated media from 3T3‐L1 cells to analyze inflammatory mediators and signaling pathways. PT significantly enhanced glycerol release and inhibited the adipogenesis‐related transcription factors. PT also promoted phosphorylation of AMP‐activated protein kinase and increased activity of adipose triglyceride lipase and hormone‐sensitive lipase. PT suppressed the nuclear transcription factor kappa‐B and mitogen‐activated protein kinase pathways when RAW 264.7 cells were cultured in differentiated media from 3T3‐L1 cells. PZ improved lipolysis and inhibited the macrophage inflammatory response less effectively than PT.
Conclusion: This study suggests that PT is more effective than PZ at increasing lipolysis in adipocytes. In addition, PT also suppresses inflammatory response in macrophage that is stimulated by differentiated media from 3T3‐L1 cells.
Panicum miliaceum L.
The dietary intake of whole grains is known to Reduce the incidence of chronic diseases such as Obesity, Diabetes, cardiovascular disease, and cancer. To investigate whether there are Anti-Adipogenic activities in various Korean cereals, we assessed water extracts of nine cereals.
The results showed that treatment of 3T3-L1 Adipocytes with Sorghum bicolor L. Moench, Setaria italica Beauvois, or Panicum miliaceum L. extract significantly Inhibited Adipocyte Differentiation, as determined by measuring oil red-O staining, triglyceride accumulation, and glycerol 3-phosphate dehydrogenase activity. Among the nine cereals, P. miliaceum L. showed the highest Anti-Adipogenic activity. The effects of P. miliaceum L. on mRNA expression of peroxisome proliferator-activated receptor-γ, sterol regulatory element-binding protein 1, and the CCAAT/enhancer binding protein-α were evaluated, revealing that the extract significantly decreased the expression of these genes in a dose-dependent manner. Moreover, P. miliaceum L. extract changed the ratio of monounsaturated fatty acids to saturated fatty acids in Adipocytes, which is related to biological activity and cell characteristics.
Pelargonidin (Pelargonium hortorum)
Pelargonidin Suppresses Adipogenesis in 3T3-L1 cells through Inhibition of PPAR-γ signaling pathway
Furthermore, pelargonidin treatment led to decrease in glucose consumption. Western blot assay illustrated that the expression of PPAR-γ was suppressed to 63.25%, 47.52%, and 21.23% after incubation with 5 μM, 10 μM, and 20 μM pelargonidin when compared with DMSO group. Then, we measured the expression of some target proteins of PPAR-γ, and found that pelargonidin decreased the expressions of HMGCR, LPL, Glut4, and A-FABP. Besides, the result of Luciferase Reporter Assay indicated that pelargonidin Inhibited PPAR-γ transcription activity. These results indicated that pelargonidin exerts Anti-Adipogenic activity in 3T3-L1 cells through Inhibition of PPAR-γ signaling pathway, and pelargonidin could be used as a potential Anti-Obesity agent.
Petalonia binghamiae (brown algae)
It also Inhibited the mitotic clonal expansion process of Adipocyte Differentiation, and it Inhibited insulin-stimulated uptake of glucose into mature 3T3-L1 Adipocytes by reducing phosphorylation of insulin receptor substrate-1. In rats with high-fat diet (HFD)-induced Obesity, PBEE exhibited potent Anti-Obesity effects. In this animal model, increases in body weight and fat storage were suppressed by the addition of PBEE to the drinking water at 500 mg/L for 30 days.
PBEE supplementation Reduced serum levels of glutamic pyruvic and glutamic oxaloacetic transaminases and increased the serum level of high-density lipoprotein cholesterol. Moreover, it significantly decreased the accumulation of lipid droplets in liver tissue, suggesting a protective effect against HFD-induced hepatic steatosis. Taken together, these data demonstrate that PBEE Inhibits Preadipocyte Differentiation and Adipogenesis in cultured cells and in rodent models of Obesity.
Peucedanum japonicum
Results: Pteryxin dose dependently suppressed TG content in both 3T3-L1 Adipocytes (by 52.7%, 53.8%, and 57.4%, respectively; P < 0.05) and HepG2 hepatocytes (by 25.2%, 34.1%, and 27.4%, respectively; P < 0.05). Sterol regulatory element-binding protein-1 (SREBP-1c), fatty acid synthase (FASN), and acetyl-coenzyme A carboxylase-1 (ACC1) were down-regulated in pteryxin-treated 3T3-L1 Adipocytes (by 18%, 36.1%, and 38.2%, P < 0.05) and HepG2 hepatocytes (by 72.3%, 62.9%, and 38.8%, respectively; P < 0.05). The Adipocyte size marker gene, paternally expressed gene1/mesoderm specific transcript (MEST) was down-regulated (by 42.8%; P < 0.05), and hormone-sensitive lipase, a lipid catabolizing gene was up-regulated (by 15.1%; P < 0.05) in pteryxin-treated Adipocytes. The uncoupling protein 2 (by 77.5%; P < 0.05) and adiponectin (by 76.3%; P > 0.05) were up-regulated due to pteryxin.
Conclusion: Our study demonstrated that pteryxin in PJT plays the key role in regulating the lipid metabolism-related gene network and improving energy production in vitro. Thus, the results suggest pteryxin as a new natural compound to be used as an AntiObesity drug in the pharmaceutical industry.
Phyllanthus emblica
Results: Digallic acid was identified as a major component in the PEFE. The IC50 values of digallic acid and PEFE were found to be 3.82 µg/ml and 21.85 µg/ml respectively. PEFE and digallic acid showed significant anti-lipolytic activity compared to the standard drug orlistat. In the mature Adipocytes, PEFE significantly decreased triglyceride accumulation by downregulating adiponectin, PPARγ, cEBPα, and FABP4 respectively. We further analyzed the expression of apoptosis related genes upon PEFE treatment. Apoptotic process initiated through upregulation of BAX and downregulation of BCL2 resulting in an increased caspase-3 activity. In addition, we have also confirmed the apoptosis and DNA fragmentation in 3T3-L1 cells using Hoechst, PI and TUNEL assays.
Conclusion: PEFE negatively regulates Adipogenesis by initiating fat cell apoptosis and therefore it can be considered as a potential herbal medicinal product for treating Obesity.
Piperine (Black Pepper)
Piperine Inhibits Adipogenesis by Antagonizing PPARγ Activity in 3T3-L1 cells
This study investigated the AntiAdipogenic activity of black pepper extract and its constituent piperine in 3T3-L1 preadipocytes as well as the underlying molecular mechanisms. Both black pepper extract and piperine, without affecting cytotoxicity, strongly Inhibited the Adipocyte Differentiation of 3T3-L1 cells. The mRNA expression of the master Adipogenic transcription factors, PPARγ , SREBP-1c, and C/EBPβ, was markedly decreased. Intriguingly, mRNA levels of PPARγ target genes were also down-regulated.
Moreover, a luciferase reporter assay indicated that pipierine significantly represses the rosiglitazone-induced PPARγ transcriptional activity. Finally, GST-pull down assays demonstrated that piperine disrupts the rosiglitazone-dependent interaction between PPARγ and coactivator CBP. Genome-wide analysis using microarray further supports the role of piperine in regulating genes associated with lipid metabolism.
Overall, these results suggest that piperine, a major component of black pepper, attenuates fat cell Differentiation by down-regulating PPARγ activity as well as suppressing PPARγ expression, thus leading to potential treatment for Obesity-related diseases.
Platycodin D (Platycodi Radix)
Treatment with platycodin D also resulted in a reduction of Peroxisome proliferator-activated receptor(PPAR)gamma expression and its binding to target DNA sequence. Among the various upstream regulators of PPARgamma, the expression of Kruppel-like factor(KLF)2, an anti-adipogenic factor, was significantly upregulated following platycodin D treatment. When the upregulation of KLF2 was inhibited by KLF2 siRNA, the expression and binding of PPARgamma to its target sequence were significantly recovered under these conditions.
The results of this study suggested that anti-adipogenic effect of platycodin D involves the upregulation of KLF2 and subsequent downregulation of PPARgamma.
Results: PD treatment attenuated body weight gain, suppressed white adipose tissue weight and improved obesity-related serum parameters in db/db mice. Two major adipogenic factors, peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer binding protein α (C/EBPα) were decreased by PD treatment in WAT of db/db mice, 3T3-L1 adipocytes and hAMSCs. In BAT of db/db mice and primary cultured brown adipocytes, PD treatment elevated the expressions of uncoupled protein 1 (UCP1) and peroxisome proliferator-activated receptor γ coactivator 1 α (PCG1α), the key regulators of BAT-associated thermogenesis. In addition, PD activated AMPKα both in vivo and in vitro. However, when AMPK was inhibited by compound C, PD treatment failed to suppress adipogenic factors and increase thermogenic factors.
Key findings: During the adipocyte differentiation of 3 T3-L1 cells, members of the WNT/β-catenin pathway were normally down-regulated, whereas platycodin D significantly reinstated the WNT/β-catenin pathway. The mRNA and protein expressions of disheveled (DVL) 2, which stabilize β-catenin, were increased by platycodin D treatment, but the protein level of AXIN, which induces the degradation of β-catenin, was decreased in platycodin D-treated cells. The nuclear level of β-catenin was normally down-regulated during adipogenesis, but platycodin D treatment led to the accumulation of β-catenin in the nucleus which resulted in the up-regulation of its target genes, cyclin D (CCND) 1 and peroxisome proliferator-activated receptor gamma (PPAR)γ. The anti-adipogenic effects of platycodin D were significantly attenuated in β-catenin siRNA-transfected cells compared with those of control siRNA-transfected cells. β-catenin siRNA transfection significantly recovered the levels of PPARγ, CCAAT/enhancer binding protein (C/EBP)α and fatty acid binding protein (FABP)4 as well as intracellular lipid droplet formation, all of which were reduced by platycodin D treatment.


Pluchea indica
The Effect of Pluchea indica (L.) Less. Tea on Adipogenesis in 3T3-L1 Adipocytes and Lipase Activity
Polygonum cuspidatum
Background: Obesity causes metabolic disease and is a serious health problem around the world. Polygonum cuspidatum (POCU1b) has been used clinically for the treatment of constipation, gallstones, hepatitis, and inflammation in East Asian countries. The principal aim of this study was to investigate for the first time whether the extract of Polygonum cuspidatum (POCU) biologically affects adipogenesis in 3 T3-L1 preadipocytes.
Methods: Fractions (n-hexan, ethyl acetate, n-butanol, and water) of POCU ethanol extract were evaluated in vitro for their inhibitory activities on pancreatic lipase. Of the fractions, the n-butanol of POCU ethanol extract (POCU1b) was examined anti-obesity activity in 3 T3-L1 preadipocytes. To examine the inhibitory effect of POCU1b on adipogenesis, 3 T3-L1 preadipocytes were treated every the other day with POCU1b at various concentrations (0 ~ 25 μg/mL) for twelve days. Oil-red O staining and triglyceride content assay were performed to determine the lipid accumulation. The expression of mRNA and proteins associated lipid accumulation was measured using RT-PCR and Western blotting analysis. We also examined the effect of POCU1b on level of phosphorylated AMP-activated protein kinase (pAMPK) in 3 T3-L1 preadipocytes with POCU1b at various concentrations during adipocyte differentiation.
Results: POCU1b exhibited the most pronounced inhibitory effects on pancreatic lipase activity. We found that POCU1b inhibited adipocyte differentiation in 3 T3-L1 preadipocytes in a dose-dependent manner, as evidenced by the reduced formation of lipid droplets and decreased glycerol-3-phosphate dehydrogenase (GPDH) activity. We also showed that the expression levels of adipocyte differentiation-related protein (ADRP) and perilipin (a protein that coats lipid droplets in adipocytes) were both reduced after POCU1b treatment. Peroxisome proliferator-activated receptor-gamma (PPAR-γ) and CCAAT/enhancer-binding protein-alpha (C/EBP-α) proteins, both major adipogenic transcription factors, were markedly reduced by POCU1b. Moreover, ADRP, perilipin, C/EBP-α, and PPAR-γ mRNA levels were also reduced by POCU1b. Levels of phosphorylated AMP-activated protein kinase (pAMPK) were elevated after POCU1b treatment (5, 10, and 25) in a dose-dependent manner.
Conclusions: Taken together, these results suggest that the anti-obesity effects of POCU1b involve the inhibition of pancreatic lipase activity and adipogenesis via the down-regulation of lipid accumulation.
Prieurianin (Turraea obtusifolia)
The global increase in the incidence of obesity has emerged as one of the most serious public health risks in recent years. Despite the enormity of the obesity pandemic, there are currently only two FDA-approved therapies for its treatment and these drugs exhibit modest efficacy and have limiting side effects. Prieurianin is a plant limonoid product that deters feeding in insect larvae. We investigated in this study the effects of prieurianin on weight loss and adipogenesis.
Our results showed that prieurianin causes weight loss by reducing energy intake in obese mice on high-calorie diet. We also found that prieurianin is anti-adipogenic in cultured preadipocytes and adipocytes by inhibiting proliferation and differentiation of preadipocytes into adipocytes, and induces either dedifferentiation or delipidation of mature adipocytes. Whether prieurianin can potentially be used for obesity treatment in human warrants further investigation.
Characterization of the Anti–Obesity and Anti-Adipogenic Effects of the Limonoid Prieurianin
However, we found that camptothecin, topoisomerase I inhibitor, does not accumulate in HDL or in other lipoprotein subfractions, thus ruling out the possibility of PLTP mediating the transfer of camptothecin into HDL for liver metabolism. The limonoid prieurianin, like topoisomerase I inhibitors, has also been shown to dose dependently increase PLTP mRNA and proteins levels, and here we show that prieurianin causes weight loss by reducing food intake in morbidly obese mice and in mice on high-calorie diet. Additionally, prieurianin is anti-adipogenic and (i) inhibits the proliferation and differentiation of preadipocytes into adipocytes, and (ii) induces either dedifferentiation or delipidation of mature adipocytes.
Gene expression profiling showed that prieurianin suppresses the expression of a number of genes involved in fat metabolism, and inhibits the transcriptional activity of the adipogenesis master regulators including the CCAAT/enhancer binding proteins (C/EBPs) and the peroxisome proliferator-activated receptor gamma (PPARγ).
Procyanidin B2 (grape seed)
Procyanidin Effects on Adipocyte -Related Pathologies
Procyanidins, a class of Flavonoids , have clear and well-defined beneficial effects against several pathologies including cardiovascular heart disease. Now, studies in vivo are revealing the effects of procyanidins against Obesity, where they Prevent Weight Gain and Adipose Tissue Mass increase, and against Diabetes and insulin resistance, where they act as antihiperglycemic agents. Several mechanisms may be responsible for these effects. One of these, due to the key role of Adipose Tissue in the development of Obesity and insulin resistance, is their effect on Adipocytes.
In this review we compile the studies that indicate a protective role for procyanidins in Obesity and insulin resistance, focusing on their effects on the Adipocyte, where procyanidins modify lipid synthesis, lipid degradation, glucose uptake, and adipose Differentiation.
Pterostilbene (Blueberry)
The aim of this work was to study the effects of garcinol and pterostilbene on cell proliferation and Adipogenesis in 3T3-L1 cells. The results showed that garcinol and pterostilbene decreased the cell population growth and caused cell cycle arrest at the G2/M phase in 3T3-L1 preadipocytes. During Adipocyte Differentiation, both garcinol and pterostilbene had Inhibitory effects on fat droplet formation and triacylglycerol accumulation. The data indicated that garcinol and pterostilbene could Inhibit the glycerol-3-phosphate dehydrogenase (GPDH) activity by 97.8 and 61.5%, respectively, as compared to the control. Both garcinol and pterostilbene significantly attenuated the protein expressions of PPARγ and C/EBPα during 3T3-L1 Adipocyte Differentiation.
Moreover, garcinol and pterostilbene caused an Inhibition of lipid accumulation in the 3T3-L1 Adipocyte Differentiation phase. Garcinol and pterostilbene also significantly up-regulated the gene expression of adiponectin as well as down-regulated the gene expressions of leptin, resistin, and fatty acid synthase (FAS) in 3T3-L1 Adipocyte Differentiation. In 3T3-L1 Adipocytes , garcinol significantly down-regulated the protein expressions of PPARγ and FAS as well as up-regulated the protein expressions of adipose triglyceride lipase (ATGL) and adiponectin. Garcinol also significantly up-regulated the gene expression of adiponectin as well as down-regulated the gene expressions of leptin and FAS. These results suggest that garcinol and pterostilbene have Anti-Adipogenic effects on preadipocytes and Adipocytes.
Punicalagin (pomegranate)
Fatty acid synthase (FAS) has been recognized as a potential therapeutic target for obesity. In this study, for the first time, the inhibitory effect of pomegranate husk extract, punicalagin and ellagic acid on FAS was investigated. We found them potently inhibiting the activity of FAS with half-inhibitory concentration values (IC50) of 4.1 μg/ml (pomegranate husk extract), 4.2 μg/ml (4.50 μM, punicalagin) and 1.31 μg/ml (4.34 μM, ellagic acid), respectively. Moreover, they all exhibited time-dependent inactivation of FAS. Punicalagin and ellagic acid inhibited FAS with different mechanisms compared to previously reported inhibitors, through inactivating acetyl/malonyl transferase and β-ketoacyl synthase domains, respectively.
Additionally, 100 μg/ml pomegranate husk extract, 5.24 μg/ml (5 μM) punicalagin and 4.5 μg/ml (15 μM) ellagic acid effectively reduced lipid accumulation inside FAS over-expressed 3T3-L1 adipocytes. Since FAS plays a key role in the biosynthesis pathway of fatty acid, these findings suggest that pomegranate husk extract, punicalagin and ellagic acid have potential in the prevention and treatment of obesity.
Results: Pomegranate juice, ellagic acid, punicalagin and urolithin A were able to inhibit lipase, α-glucosidase and dipeptidyl peptidase-4. Furthermore, all tested compounds but significantly the metabolite urolithin A displayed anti-adipogenic properties in a dose-dependent manner as they significantly reduced TG accumulation and gene expression related to adipocyte formation such as adiponectin, PPARγ, GLUT4, and FABP4 in 3T3-L1 adipocytes.
Conclusion: These results may explain from a molecular perspective the beneficial effects and traditional use of pomegranate in the prevention of metabolic-associated disorders such as obesity, diabetes and related complications.
Methods and results: The effect of PCG on adipogenesis is examined using Oil red O staining. The effects and mechanism of action of PCG on inflammatory responses are determined in adipocyte-conditioned medium (ACM)-cultured macrophages, a cell-to-cell contact system, and a transwell system. The effects of PCG on obesity and obesity-induced inflammatory/oxidant responses are examined in high-fat diet (HFD)-fed mice. PCG effectively suppresses lipid accumulation in adipocytes and adipocyte-induced inflammatory responses in adipocyte-macrophage co-culture systems. Small interfering RNA (siRNA) transfection indicates that the PCG-mediated anti-inflammatory effect is exerted via the nuclear factor erythroid 2-related factor 2/Kelch-like ECH-associated protein 1(Nrf2/Keap1) pathway. PCG administration results in a significant reduction in body and white adipose tissue (WAT) weights. PCG favorably regulates pro- and anti-inflammatory cytokines, downregulating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). Immunohistochemical (IHC) analysis demonstrates that PCG differentially modulates the distribution of complement component 3 receptor 4 subunit (CD11c) and cluster of differentiation 206 (CD206). PCG regulates the level of antioxidant and oxidant molecules by activating Nrf2/Keap1 signaling.
Conclusions: PCG ameliorates obesity and obesity-induced inflammatory responses via activation of Nrf2/Keap1 signaling, suggesting that PCG has potential as an oral agent to control obesity-mediated diseases.
Beneficial effects of pomegranate peel extract and probiotics on pre-Adipocyte Differentiation
The beneficial effects of pomegranate are due to the ellagitannins and anthocyanins content, which are protective toward a wide variety of diseases including inflammatory diseases. Many investigators have reported that pomegranate waste (peel and seeds) extracts, made from waste product of industrial processing, show free radical scavenger and a potent antioxidant capacity. Pomegranate extracts (PEs) were also reported to possess noteworty antibacterial, antiviral, hypolipidemic, and anti-inflammatory bioactivities thanks to the polyphenolic compounds content, which includes punicalagins, gallic acid, and ellagic acid derivatives.
The focus of the present manuscript was to study the prebiotic potentiality of a PE, soluble in water, and characterized through HPLC–PDA–ESI/MSn for its phenolic content. Moreover, since it has been reported that pomegranate extracts decreased the level of lipids in the blood and that a number of probiotic strains have been shown to affect adipogenesis in cell culture, this study was also performed to test the in vitro effects of PE and probiotic L. rhamnosus GG ATCC 53103 strain (LGG) on 3T3-L1 cell line.
Quercetin
Quercetin‐rich onion peel extract Suppresses Adipogenesis by down‐regulating Adipogenic transcription factors and gene expression in 3T3‐L1 Adipocytes
Results: Without affecting cell viability, both OPE and quercetin averted adipogenesis, as characterized by dose-dependent decreases in intracellular triglyceride content and glycerol 3-phosphate dehydrogenase activity, but the effect was more pronounced with OPE than with quercetin. The mRNA expression levels of key adipogenic genes such as PPARγ, C/EBPα, FABP4, aP2 and LPL were decreased in a dose-dependent manner by both OPE and quercetin.
Conclusion: The results indicate that OPE treatment significantly prevents intracellular lipid accumulation via hyperactivation of genes regulating lipolysis as compared with quercetin alone.
In mature adipocytes resveratrol and quercetin at 100 microM individually decreased viability by 18.1+/-0.6% (p<0.001) and 15.8+/-1% (p<0.001) and increased apoptosis (100 microM) by 120.5+/-8.3% (p<0.001) and 85.3+/-10% (p<0.001) at 48 h, respectively. Combinations of resveratrol and quercetin further decreased viability (73.5+/-0.9%, p<0.001) and increased apoptosis (310.3+/-9.6%, p<0.001) more than single compounds alone. The combination of resveratrol and quercetin at 100 muM increased release of cytochrome c from mitochondria to cytosol and decreased ERK 1/2 phosphorylation. Taken together, our data indicate that combinations of resveratrol and quercetin can exert potential anti-obesity effects by inhibiting differentiation of preadipocytes and inducing apoptosis of mature adipocytes.
Quercetin pretreatment reduced the ROS generation stimulated by either CSE or H(2)O(2) in preadipocyte OFs. CSE and H(2)O(2) stimulated adipocyte differentiation in cultured OFs. The addition of quercetin (50 or 100 μM) suppressed adipogenesis. Quercetin also suppressed ROS generation in differentiating OFs during adipogenesis stimulated by CSE and H(2)O(2). Additionally, the expressions of PPARγ, C/EBPα, and C/EBPβ proteins were reduced in the quercetin-treated OFs. Quercetin also reduced the CSE- and H(2)O(2)-induced upregulation of ROS and HO-1 protein in differentiated OFs and preadipocyte OFs. As shown in this study, quercetin inhibited adipogenesis by reducing ROS in vitro, supporting the use of quercetin in the treatment of GO.
Quercetin prevents Adipogenesis by regulation of transcriptional factors and lipases in OP9 cells
The mRNA expression levels of lipases, such as adipose triglyceride lipase (ATGL), hormone sensitive lipase (HSL) and lipoprotein lipase (LPL) were also measured by RT-PCR. Quercetin significantly decreased the expression of transcription factors, including C/EBPα, PPARγ and SREBP-1c both at the protein and mRNA level. The results from the present study demonstrate that quercetin prevents adipogenesis by upregulating ATGL and HSL expression and downregulating FAS, LPL and adipocyte fatty acid-binding protein (aP2) expression, as well as the expression of transcription factors. Our data suggest that quercetin has therapeutic potential by regulating the expression of transcriptional factors and enzymes associated with adipogenesis.
Combined effects of genistein, quercetin, and resveratrol in human and 3T3-L1 Adipocytes
We also determined whether MAs responded to the combination treatment similarly to HAs. As in HAs, G+Q+R treatment decreased lipid accumulation in maturing MAs and increased apoptosis in pre- and lipid-filled mature MAs more than the responses to G, Q, and R when used separately. These results show that lower concentrations of combined treatments with several natural compounds may be useful for treatments for obesity through the suppression of adipogenesis and enhanced adipocyte apoptosis.
Q and quercetin 3-glucoronide prevented TNFα-mediated downregulation of adipogenesis during 3T3-L1 pre-adipocytes early differentiation. Together, Q capacity to promote a healthy adipose expansion enhancing angiogenesis and adipogenesis may contribute to reduced adipose hypertrophy, inflammation and IR. Consumption of diets rich in Q could be useful to counteract the adverse effects of high-fat diet-induced adipose dysfunction.
In this study, we confirmed the inhibitory effects of quercetin in adipogenesis and inflammation using a mouse model. In mice, quercetin reduced body weight (almost 40%) and suppressed expression of adipogenic, lipogenic and inflammation-related cytokines. Our data demonstrated that quercetin inhibits lipid accumulation and obesity-induced inflammation in the cell and animal models. Our study suggested that quercetin may represent a potential therapeutic agent for other metabolic disorders by regulating obesity and obesity-induced inflammation.
Raspberry ketone
Heme oxygenase-1 mediates anti–Adipogenesis effect of raspberry ketone in 3T3-L1 cells
Results: RK (300–400 µM) strongly inhibited lipid accumulation during 3T3-L1 preadipocyte differentiation into adipocytes. RK reduced the CCAAT/enhancer-binding protein-α (C/EBP-α), peroxisome proliferation-activated receptor-γ (PPAR-γ), fatty acid synthase (FAS), and fatty acid-binding protein 4 (FABP4) expressions and increased heme oxygenase-1 (HO-1), Wnt10b, and β-catenin expressions in 3T3-L1 adipocytes. Additionally, RK inhibited lipid accumulation, and adipogenic transcription factor and lipogenic protein expressions were all decreased by inhibiting HO-1 or β-catenin using tin protoporphyrin (SnPP) or β-catenin short-interfering RNA (siRNA), respectively. Furthermore, Wnt10b and β-catenin expressions were negatively regulation by SnPP.
Conclusion: RK may exert anti-adipogenic effects through modulation of the HO-1/Wnt/beta-catenin signaling pathway.
Context: Raspberry ketone (RK) is a natural phenolic compound of red raspberry. The dietary intake of RK has been reported to exert anti-obese actions and alter the lipid metabolism in vivo and human studies.
Objective: To elucidate a possible mechanism for anti-obese actions of RK, the effects of RK on the adipogenic and lipogenic gene expression in 3T3-L1 adipocytes were investigated.
Materials and methods: 3T3-L1 maturing pre-adipocytes were treated from day 2 to day 8 of differentiation and mature adipocytes for 24 h on day 12 with 1, 10, 20, and 50 μM of RK. Triacylglycerols were assessed by spectrophotometry and gene expression by quantitative real-time polymerase chain reaction (qRT-PCR).
pathway including peroxisome proliferator-activated receptor-γ (PPARγ) and CCAAT enhancer binding protein-α (C/EBPα), which led to further down-regulation of adipocyte fatty acid-binding protein-2 (aP2). In addition, treatment with 10 μM of RK also reduced mRNA levels of lipogenic genes such as acetyl-CoA carboxylase-1 (ACC1), fatty acid synthase (FASN), and stearoyl-CoA desaturase-1 (SCD1). In mature adipocytes, RK increased the transcriptional activities of genes involved in lipolysis and the oxidative pathways including adipose triglyceride lipase (ATGL), hormone sensitive lipase (HSL), and carnitine palmitoyl transferase-1B (CPT1B).
Discussion and conclusion: These findings suggest that RK holds great promise for an herbal medicine with the biological activities altering the lipid metabolism in 3T3-L1 adipocytes.
This study aimed to determine the antiobesity effects of raspberry ketone (RK), one of the major aromatic compounds contained in raspberry, and its underlying mechanisms. During adipogenesis of 3T3-L1 cells, RK (300 μM) significantly reduced lipid accumulation and downregulated the expression of CCAAT/enhancer-binding protein α (C/EBPα), peroxisome proliferation-activated receptor γ (PPARγ), fatty acid-binding protein 4 (FABP4), and fatty acid synthase (FAS). RK also reduced the expression of light chain 3B (LC3B), autophagy-related protein 12 (Atg12), sirtuin 1 (SIRT1), and phosphorylated-tuberous sclerosis complex 2 (TSC2), whereas it increased the level of p62 and phosphorylated-mammalian target of rapamycin (mTOR). Daily administration of RK decreased the body weight (ovariectomy [Ovx] + RK, 352.6 ± 5 vs Ovx, 386 ± 5.8 g; P < 0.05), fat mass (Ovx + RK, 3.2 ± 0.05 vs Ovx, 5.0 ± 0.4 g; P < 0.05), and fat cell size (Ovx + RK, 6.4 ± 0.6 vs Ovx, 11.1 ± 0.7 × 103 μm2; P < 0.05) in Ovx-induced obesity in rats.
The expression of PPARγ, C/EBPα, FAS, and FABP4 was significantly reduced in the Ovx + RK group compared with that in the Ovx group. Similar patterns were observed in autophagy-related proteins and endoplasmic reticulum stress proteins. These results suggest that RK inhibited lipid accumulation by regulating autophagy in 3T3-L1 cells and Ovx-induced obese rats.
Additionally, these effects of RK were reversed by the HO-1 inhibitor SnPP (20 μM). In addition, RK (160 mg/kg, gavage, for 8 weeks) significantly reduced body weight gain (Ovx+RK, 191.8 ± 4.6 g vs. Ovx, 223.6 ± 5.9; P < .05), food intake, the amount of inguinal adipose tissue (Ovx+RK, 9.05 ± 1.1 g vs Ovx, 12.9 ± 0.92 g; P < .05) and the size of white adipocytes in Ovx rats. Moreover, compared to expression in the Ovx group, the levels of browning-specific proteins were significantly higher and the levels of autophagy-related proteins were significantly lower in the Ovx+RK group. Therefore, this study elucidated the mechanism associated with RK-induced WAT browning and thus provides evidence to support the clinical use of RK for obesity treatment.
Raspberry ketone promotes the Differentiation of C3H10T1/2 stem cells into osteoblasts
RK in the presence of ATRA increased alkaline phosphatase (ALP) activity in a dose-dependent manner. RK in the presence of rhBMP-2 also increased ALP activity. RK in the presence of ATRA also increased the levels of mRNAs of osteocalcin, α1(I) collagen, and TGF-βs (TGF-β1, TGF-β2, and TGF-β3) compared with ATRA only. RK promoted the differentiation of C3H10T1/2 stem cells into osteoblasts. However, RK did not affect the inhibition of early-stage adipocyte differentiation. Our results suggest that RK enhances the differentiation of C3H10T1/2 stem cells into osteoblasts, and it may promote bone formation by an action unrelated to adipocyte differentiation.
Raspberry ketone attenuates high-fat diet-induced Obesity by improving metabolic homeostasis in rats
Results: Body weight, blood glucose, insulin, total lipids, triacylglycerols, total cholesterol and low-density lipoprotein cholesterol were increased in high-fat diet fed rats. These high fat diet-induced changes were attenuated by treatment with raspberry ketone. High-density lipoprotein cholesterol was decreased in highfat diet fed rats but increased in rats treated with raspberry ketone. Molecular investigations showed induction of gene expression of C/EBP-δ, FAS, ACC, CPT1A and inhibition of gene expression of PPAR-α and HSL in high-fat diet fed rats as compared with control. Raspberry ketone treament reversed these changes except CPT1A.
Conclusions: Raspberry ketone can prevent obesity induced by a high-fat diet in rats by induction of the expression of enzymes, controlling lipolysis and fatty acids β oxidation as well as inhibition of gene expressions of adipogenic factors.
Regarding the structure-activity relationship, the higher concentration of RK induced slight modulation of the shape of the active site as monitored by hydrophobic exposure. The tertiary conformational change was linked to RK inhibition, and mostly involved regional changes of the active site. Our study provides insight into the functional role of RK due to its structural property of a hydroxyphenyl ring that interacts with the active site of α-glucosidase. We suggest that similar hydroxyphenyl ring compounds targeting the key residues of the active site might be potential α-glucosidase inhibitors.
Results: RK is rapidly absorbed (Tmax ≈ 15 min), and bioconverted into diverse metabolites in mice. Total bioavailability (AUC0-12 h ) is slightly lower in females than males (566 vs 675 nmol mL-1 min-1 ). Total bioavailability in obese mice is almost doubled that of control mice (1197 vs 679 nmol mL-1 min-1 ), while peaking times and elimination half-lives are delayed. Higher levels of RK and major metabolites are found in WAT of the obese than normal-weight animals.
Conclusions: RK is highly bioavailable, rapidly metabolized, and exhibits significantly different pharmacokinetic behaviors between obese and control mice. Lipid-rich tissues, especially WAT, can be a direct target of RK.
Rehmannia glutinosa
Rehmannia Inhibits Adipocyte Differentiation and Adipogenesis
Rehmannia glutinosa, a Traditional Chinese Medicine (TCM), has been used to increase physical strength. Here, we report that Rehmannia glutinosa extract (RE) inhibits adipocyte differentiation and adipogenesis. RE impairs differentiation of 3T3-L1 preadipocytes in a dose-dependent manner. At the molecular level, treatment with RE inhibits expression of the key adipocyte differentiation regulator C/EBPβ, as well as C/EBPα and the terminal marker protein 422/aP2, during differentiation of preadipocytes into adipocytes. Additionally, RE inhibits the mitotic clonal expansion (MCE) process of adipocyte differentiation, and RE prevents localization of C/EBPβ to the centromeres. RE also prevents high fat diet (HFD) induced weight gain and adiposity in rats. Taken together, our results indicate that Rehmannia glutinosa extract inhibits preadipocyte differentiation and adipogenesis in cultured cells and in rodent models of obesity.
Anti-Adipogenic effects of the traditional herbal formula Dohongsamul-tang in 3T3-L1 Adipocytes
Results: Oil Red O staining showed that DHSMT markedly reduced fat accumulation without affecting cell cytotoxicity. DHSMT also significantly decreased accumulation of triglyceride and adipokines such as leptin, adiponectin, resistin and PAI-1 compared with fully differentiated adipocytes. Furthermore, our results found that DHSMT significantly suppressed the adipocyte differentiation by downregulating adipogenic-specific transcriptional factors such as peroxisome proliferator-activated receptor gamma (PPARγ), CCAAT/enhancer binding proteins alpha (C/EBPα) and fatty acid binding protein 4 (FABP4) in adipocytes.
Conclusions: Taken together, our findings provide that DHSMT has potential for treatment and prevention of obesity or MS related to BSS.
Resveratrol
Resveratrol potentiates genistein’s AntiAdipogenic and proapoptotic effects in 3T3-L1 Adipocytes
G25 + R25 decreased lipid accumulation by 77.9 +/- 3.4% (P < 0.001). Lipolysis assay revealed that neither G25 nor R25 induced lipolysis, whereas G25 + R25 significantly increased lipolysis by 25.5 +/- 4.6%. The adipocyte-specific proteins PPARgamma and CCAAT/enhancer binding protein-alpha were downregulated after treatment with G + R, but no effect was observed with individual compounds. These results indicate that G and R in combination produce enhanced effects on inhibiting adipogenesis, inducing apoptosis, and promoting lipolysis in 3T3-L1 adipocytes. Thus, the combination of G and R is more potent in exerting antiobesity effects than the individual compounds.
Resveratrol significantly reversed the HFD-induced up-regulation of galanin-mediated signaling molecules (GalR1/2, PKCδ, Cyc-D, E2F1, and p-ERK) and key adipogenic genes (PPARγ2, C/EBPα, SREBP-1c, FAS, LPL, aP2, and leptin) in the epididymal adipose tissues of mice. Furthermore, resveratrol significantly attenuated the HFD-induced up-regulation of pro-inflammatory cytokines (TNFα, IFNα, IFNβ, and IL-6) and their upstream signaling molecules (TLR2/4, MyD88, Tirap, TRIF, TRAF6, IRF5, p-IRF3, and NF-κB) in the adipose tissues of mice. The results of this study suggest that resveratrol inhibits visceral adipogenesis by suppressing the galanin-mediated adipogenesis signaling cascade. It may also attenuate cytokine production in the adipose tissue by repressing the TLR2- and TLR4-mediated pro-inflammatory signaling cascades in HFD-fed mice.
Potential miRNA involvement in the Anti-Adipogenic effect of resveratrol and its metabolites
Results: Of the miRNAs analyzed only miR-155 was modified after resveratrol and glucuronide metabolite treatment. In transfected cells with anti-miR-155, RSV did not reduce cebpβ gene and protein expression. 3S decreased gene expression of creb1, klf5, srebf1 and lxrα.
Conclusions: While RSV and glucuronide metabolites exert their inhibitory effect on adipogenesis through miR-155 up-regulation, the anti-adipogenic effect of 3S is not mediated via miRNAs.
Resveratrol inhibited insulin signaling pathway in the early phase of adipogenesis. Furthermore, we revealed an inhibitory effect of resveratrol on insulin receptor (IR) activity, and this is likely through a direct physical interaction between resveratrol and IR. The antiadipogenic effect of resveratrol is through inhibition of the MCE and IR-dependent insulin signaling pathway in the early phase of adipogenesis.
Resveratrol induces apoptosis and Inhibits Adipogenesis in 3T3‐L1 Adipocytes
In order to understand the anti-adipogenic effects of resveratrol, maturing 3T3-L1 preadipocytes were treated with 25 microM resveratrol and the change in the expression of several adipogenic transcription factors and enzymes was investigated using real-time RT-PCR. Resveratrol down-regulated the expression of PPAR gamma, C/EBP alpha, SREBP-1c, FAS, HSL, LPL and up-regulated the expression of genes regulating mitochondrial activity (SIRT3, UCP1 and Mfn2). These results indicate that resveratrol may alter fat mass by directly affecting cell viability and adipogenesis in maturing preadipocytes and inducing apoptosis in adipocytes and thus may have applications for the treatment of obesity.
Results: Epididymal fat depots were reduced in mice drinking phenelzine alone or with resveratrol. No limitation of body weight gain or body fat content was observed in the groups drinking resveratrol or phenelzine, separately or in combination. The altered glucose tolerance and the increased fat body composition of VHFD-fed mice were not reversed by resveratrol and/or phenelzine. Such lack of potentiation between resveratrol and phenelzine prompted us to verify in vitro their direct effects on mouse adipocytes. Both molecules inhibited de novo lipogenesis, but did not potentiate each other at 10 or 100 μM. Only resveratrol inhibited hexose uptake in a manner that was not improved by phenelzine.
Conclusions: Phenelzine has no interest to be combined with low doses of resveratrol for treating/preventing obesity, when considering the VHFD mouse model.
Certain flavonoids have been shown to have specific effects on biochemical and metabolic functions of adipocytes. In this study, we investigated the effects of combinations of resveratrol and quercetin on adipogenesis and apoptosis in 3T3-L1 cells. In maturing preadipocytes resveratrol and quercetin at 25 microM individually suppressed intracellular lipid accumulation by 9.4+/-3.9% (p<0.01) and 15.9+/-2.5%, respectively, (p<0.001). The combination of resveratrol and quercetin at the same dose, however, decreased lipid accumulation by 68.6+/-0.7% (p<0.001). In addition, combinations of resveratrol and quercetin at 25 microM significantly decreased the expression of peroxisome proliferators-activated receptor gamma (PPAR gamma) and CCAAT/enhancer-binding protein (C/EBP)alpha, both of which act as key transcription factors. In mature adipocytes resveratrol and quercetin at 100 microM individually decreased viability by 18.1+/-0.6% (p<0.001) and 15.8+/-1% (p<0.001) and increased apoptosis (100 microM) by 120.5+/-8.3% (p<0.001) and 85.3+/-10% (p<0.001) at 48 h, respectively.
Moreover, combined G, E, and/or R attenuated protein expressions of fatty acid binding protein 4 and perilipin, two PPAR-γ/C/EBP-α downstream molecules in fat drop development in a very similar pattern, in inhibiting differentiation in preadipocytes. This combined antiadipogenic effect of G + E + R is additive, not synergistic according to our results and the Median-Effect Principle. In addition, we found that a lower concentration (15 μM) of G, E, and/or R is required in HPAs than the concentration (30 μM) needed in 3T3-L1 cells, to exert the combined antiadipogenic effect. These data suggest that combinations of G, E, and/or R intake or soy bean, green tea, and/or grape simultaneous consumption may prevent obesity in human being.
Adipocyte Differentiation plays a pivotal role in the progression of Obesity which is a major risk factor for several diseases such as Diabetes, hypertension and coronary heart disease. In this study, the Inhibitory effect of rhamnetin, a flavonoid compound, on Adipogenesis in 3T3-L1 cells was investigated. Rhamnetin decreased the accumulation of lipid droplets, and Inhibited the elevation of triglyceride content in the Adipocytes (IC50 = 17.3 μM). The expressions of PPARγ, C/EBPα, and perilipin, Adipocyte Differentiation markers, were significantly Reduced by rhamnetin.
Triglyceride biosynthesis and clonal expansion of Adipocytes were completely Inhibited during the early stage by rhamnetin. Additionally, rhamnetin significantly decreased the expression of C/EBPβ, an early stage marker. Our results indicate that suppression of clonal expansion during the early stage of Adipogenesis by rhamnetin may be associated with Inhibition of the C/EBPβ, C/EBPα, and PPARγ pathways.
Rhein (Rhizoma Rhei)
Rhein, an Inhibit or of Adipocyte Differentiation and Adipogenesis
Rhein (RH), a compound purified from Radix et Rhizoma Rhei, has been used to alleviate liver and kidney damage. It is found that RH inhibited the differentiation of 3T3-L1 preadipocytes induced by differentiation medium in a time- and dose-dependent manner. It was revealed that RH downregulated the expression of adipogenesis-specific transcription factors PPARγ and C/EBPα, as well as their upstream regulator, C/EBPβ. Furthermore, the PPARγ target genes that are involved in adipocyte differentiation, such as CD36, aP2, acyl CoA oxidase, uncoupled protein 2, acetyl-CoA carboxylase, and fatty acid synthase, were reduced after to RH. In addition, high-fat diet-induced weight gain and adiposity were reversed by RH in C57BL/6 mice.
Consistent with the cells’ results, RH downregulated the mRNA levels of PPARγ and C/EBPα, and their downstream target genes in C57BL/6 mice. Taken together, adipocyte differentiation and adipogenesis were inhibited by RH in cultured cells and in rodent models of obesity. The evidence implied that RH was a potential candidate for preventing metabolic disorders.
The expression levels of LXR target genes were suppressed by rhein in 3T3-L1 and HepG2 cells. In white adipose tissue, muscle and liver, rhein reprogrammed the expression of LXR target genes related to adipogenesis and cholesterol metabolism. Rhein activated uncoupling protein 1 (UCP1) expression in brown adipose tissue (BAT) in wild-type mice, but did not affect UCP1 expression in LXR knockout mice. In HIB-1B brown adipocytes, rhein activated the UCP1 gene by antagonizing the repressive effect of LXR on UCP1 expression. This study suggests that rhein may protect against obesity and related metabolic disorders through LXR antagonism and regulation of UCP1 expression in BAT.
Rhein: a review of pharmacological activities
Rhein (4, 5-dihydroxyanthraquinone-2-carboxylic acid) is a lipophilic anthraquinone extensively found in medicinal herbs, such as Rheum palmatum L., Cassia tora L., Polygonum multiflorum Thunb., and Aloe barbadensis Miller, which have been used medicinally in China for more than 1,000 years.
Its biological activities related to human health are being explored actively. Emerging evidence suggests that rhein has many pharmacological effects, including hepatoprotective, nephroprotective, anti-inflammatory, antioxidant, anticancer, and antimicrobial activities. The present review provides a comprehensive summary and analysis of the pharmacological properties of rhein, supporting the potential uses of rhein as a medicinal agent.
Effect of Rhein on 3T3-L1 Preadipocyte Differentiation and Related Gene Expression
Results: The results showed that rhein(20,40,80 μM)could inhibit adipocyte differentiation and reduce intracellular TG content in a dose-dependent manner. Furthermore,rhein down-regulated the mRNA expression of C/EBPα,FAS,and PPARγ genes.
Conclusion: Rhein can inhibit the differentiation of 3T3-L1 adipocytes, and the mechanism of action may be related to reducing adipose differentiation-related gene PPARγ,C/EBPα and FAS expression.
Rhizoma Polygonati
Finally, co-transfection assays using luciferase reporter gene and reverse transcription-polymerase chain reaction (RT-PCR) analysis using peroxisome proliferator-activated receptor-γ (PPARγ) target genes indicated that kaempferol significantly repressed rosiglitazone-induced PPARγ transcriptional activity. Overall, our data suggests that kaempferol, a major component of RPF, may be beneficial in obesity, by reducing adipogenesis and balancing lipid homeostasis partly through the down-regulation of PPARγ.
Anti-Adipogenic effect of kaempferol, a component of Polygonati rhizoma
Objective: It has been reported that Polygonati rhizoma (Pr) has anti-hyperglycemia, anti-triglycemia, anti-diabetic, and anti-tumor activity. Total extract of Pr was tested to identify anti-adipogenic activity in 3T3-L1 differentiation and molecular mechanism of Pr in 3T3-L1 differentiation. Methods: Differentiation of 3T3-L1 pre-adipocyte was induced in the presence of Pr extract and kaempferol. The level of lipid accumulation was measured by Oil Red O staining. The expression of genes associated with adipocyte differentiation was measured by RT-PCR. Results: Extract of Pr and its component kaempferol reduced lipid accumulation in 3T3-L1 during adipogenesis and also reduced mRNA levels of genes associated with adipogenesis, such as adipsin, aP2, LPL, SERBP-1c and PPARγ. Conclusions: In this study, we showed that the molecular mechanism of Pr and kaempferol activity is related to regulation of PPARγ expression and activation.

Radiation is a promising technique for improving the safety and shelf-life of processed foods. In the present investigation, the degradation mechanism and bioactivity improvement of rosmarinic acid (RA) were studied in response to various gamma irradiation doses (10, 20, and 50 kGy). RA exposed to gamma irradiation at 50 kGy was completely degraded and showed an increased Inhibitory effect against 3 T3-L1 Preadipocyte compare to the parent compound. Structures of the newly generated compounds 2–4 from irradiated RA at 50 kGy were elucidated based on spectroscopic methods, including 1H nuclear magnetic resonance (NMR) and Mass spectrometry (MS). Interestingly, compounds 2 and 5 exhibited significantly enhanced Anti-Adipogenic properties in 3 T3-L1 cells compared to the original compound.


The results showed that s-petasin presented strong Anti-Adipogenic activity. Further studies illustrated that s-petasin Reduced glucose uptake. Moreover, results showed that triglyceride accumulation was Inhibited by s-petasin in differentiated 3T3-L1 cells . Western blot assay indicated that s-petasin down-regulated the expression of PPAR-γ and its target genes in a dose dependent manner. In conclusion, we isolated s-petasin from Petasites japonicus and found that it exerted Anti-Adipogenic activity against 3T3-L1 cell Differentiation through Inhibition of the expression of PPAR-γ pathway signaling.
Salicortin (Populus balsamifera)
The current study report the results of a classical bioassay-guided fractionation approach aimed at identifying the active principles responsible for the Inhibition of Adipogenesis, as measured using triglyceride accumulation in the 3T3-L1 Adipocyte model cell line. The glycosides oregonin and salicortin were isolated and identified as the respective active principles for Alnus incana and Populus balsamifera. These compounds thus offer promise as novel agents to mitigate the incidence or the progression of Obesity.

Treatment of SME significantly Inhibited ROS production and Adipocyte Differentiation that is depend on down regulation of NADPH oxidase 4 (NOX4), a major ROS generator, and peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer-binding protein alpha (C/EBPα), a key Adipogenic transcription factor. These results indicate that SME can Inhibit Adipogenesis through a Reduced ROS level that involves down-regulation of NOX4 expression or via modulation of Adipogenic transcription factor.

The brown alga, Sargassum serratifolium C. Agardh, has been reported to have high levels of meroterpenoids having anti-inflammatory, antioxidant, and anti-Diabetic activities. This study investigated the effect of ethanolic extract of S. serratifolium (ESS) on the Inhibition of Adipogenesis and lipid accumulation in 3T3-L1 preadipocytes. ESS suppressed the accumulation of lipid droplets and triglycerides (TG), while enhancing the Lipolysis of intracellular TG. We found that ESS induced cell cycle arrest at the G1/S phase via suppressing protein expression of cyclin-dependent kinases (CDK) 2, CDK4, CDK6, and cyclin E.
Moreover, ESS Reduced the expression of CCAAT/enhancer-binding protein (C/EBP) β during the early stage of Adipogenesis , leading to suppressed expression of both messenger RNA (mRNA) and protein of C/EBPα and peroxisome proliferator-activated receptor γ. As a result, ESS Reduced mRNA and protein levels of lipogenic transcription factors such as retinoid X receptor, and sterol regulatory element-binding protein 1c and their target genes, such as fatty acid synthase and stearoyl-CoA desaturase (SCD) 1. Overall, ESS Inhibited Adipocyte Differentiation in early stage and lipid accumulation during Adipogenesis through downregulation of Adipogenic and lipogenic transcription factors, providing the Anti-Obesity mechanism of ESS.

Saururus chinensis
In this study, ethanol extracts of S. chinensis significantly decrease the lipid accumulation in the 3T3-L1 cells proved by measuring triglyceride contents and Oil red O staining. The proposed mechanism of Inhibition of Adipogenesis in the 3T3-L1 cells with ethanol extracts of S. chinensis is down-regulation of transcriptional factors and Adipocyte-specific genes such CCAAT/enhancer binding protein α (C/EBPα) and Peroxisome proliferator activated receptor γ (PPARγ) in concentration dependent pattern. These results suggest that ethanol extracts of S. chinensis Inhibits adipognesis in the 3T3-L1 cells and can be used as a safe and efficient natural substance to manage Anti-Obesity.
Sanggenol F (Morus alba)
Adipose Tissue is not only a lipid storage site, but also a well-known endocrine organ. Dysfunction of Adipose Tissue is associated with irregular lipid metabolism, ectopic lipid accumulation and insulin resistance. It is proposed that modulating on Adipose Tissue is a reasonable way to ameliorate glucose and lipid metabolism. (±)-sanggenol F (SGF, purity >98.5%) was synthesized as a racemic mixture of natural (+)-sanggenol F. In this study, SGF was found to promote Adipocyte Differentiation, enhance insulin sensitivity, and upregulate beneficial adipokines expression in 3T3-L1 cells.
Furthermore, in vivo study showed that treatment with SGF for 4 weeks improved glucose metabolism, by decreasing fasting blood glucose and enhancing insulin sensitivity. It also improved lipid metabolism, with Reduced serum lipid level and ameliorated hepatic steatosis in db/db mice. During the process of target finding, we found that SGF had multiple activities of protein tyrosine phosphatase 1B Inhibition, peroxisome proliferator-activated receptor γ and peroxisome proliferator-activated receptor α agonism. These results showed the potential of SGF as a candidate for the therapy of type 2 Diabetes.
Securigera securidaca
Diabetes mellitus is associated with dysregulation of Adipose Tissue metabolism and increased level of serum lipids. In our previous work we found that Securigera securidaca decreases cholesterol level in blood of Diabetic rats. The present study was carried out to further investigate the effects of this plant on lipid metabolism, Lipolysis, and Adipogenesis, in Diabetic rats. Female Wistar rats were rendered Diabetic by intraperitoneal injection of streptozotocin. Retroperitoneal Adipose Tissue was removed from Diabetic animals after seven days of streptozotocin injection. Effect of hydroalcoholic extract of S. securidaca seeds (100–800 𝜇g/mL) on Adipose Tissue Lipolysis was evaluated in ex vivo condition. Also, to evaluate Adipogenesis , preadipocytes were isolated from Adipose Tissue and differentiated to Adipocytes in the presence of the extract.
sesamol (sesame oil)
Methods and results: In the experimental model that consisted of 3-month-old C57BL/6J mice divided into three groups with/without sesamol in the drinking water including standard diet, high fat and high fructose diet (HFFD), and HFFD with sesamol. Results demonstrated that sesamol mitigated bodyweight gain, development of insulin resistance induced by HFFD. Sesamol was found partially normalized serum and hepatic lipid contents, as well as suppressed HFFD-induced lipogenesis in liver via regulating mitochondria-related triglyceride/cholesterol metabolism genes expressions. Importantly, sesamol decreased mass and adipocyte sizes of white adipose tissues and brown adipose tissues by improving mitochondria-related genes expressions including Pgc1a and Ucp1. Moreover, sesamol was also found to reduce differentiation and mitochondrial metabolic inhibitors (oligomycin and antimycin A) stimulated lipid accumulation in 3T3-L1 adipocytes.
Conclusion: Taken together, this study provides compelling evidence that sesamol supplementation reduced adipocyte size and adipogenesis of diet-induced obesity by regulating mitochondria lipid metabolism.
Anti-Adipogenic effects of sesamol on human mesenchymal stem cells
Human mesenchymal stem cells (hMSCs) from adult bone marrow are able to differentiate into adipocytes, osteoblasts, chondrocytes and neuronal cells. Adipocytes in bone marrow are primarily responsible for the maintenance of bone structure by maintaining cell number balance with other stromal cells. However, the number of adipocytes in the bone marrow increases with age, leading to an imbalance of the bone marrow microenvironment, which results in a disruption of bone structure.
In addition, the excessive number of adipocytes in bone marrow can cause diseases, such as osteoporosis or anemia. In this study, we investigated the effect of sesamol, a major natural phenolic compound of sesame oil, on the adipogenic differentiation of hMSCs. Numerous studies have reported the anti-oxidant property of sesamol, but its effect on cell differentiation has not yet been shown. We first found that sesamol treatment during adipogenic differentiation of hMSCs reduced intracellular lipid accumulation, which was unrelated to lipolysis. Interestingly, sesamol diminished the expression of genes responsible for adipogenesis, but increased the expression of osteogenic genes.
Moreover, 76% (60/79 genes) of the sesamol-induced genes were also regulated by tert-butylhydroquinone (tBHQ), a known Nrf2 activator. We further verified that sesamol directly activated the Nrf2-mediated transcription. In addition, the Hmox1 and Ucp1 induction by sesamol was compromised in Nrf2-deleted cells, indicating the necessity of Nrf2 in the sesamol-mediated Ucp1 induction.
Together, these findings demonstrate the effects of sesamol in inducing Ucp1 and in increasing energy expenditure, further highlighting the use of the Nrf2 activation in stimulating thermogenic adipocytes and in increasing energy expenditure in obesity and its related metabolic diseases.
Results: Sesamol significantly reduced the body weight gain of obese mice and suppressed lipid accumulation in adipose tissue and liver. Sesamol also improved serum and hepatic lipid profiles, and increased insulin sensitivity. In the sesamol-treated group, the levels of serum ALT and AST decreased significantly. Furthermore, after sesamol treatment, the hepatic sterol regulatory element binding protein-1 (SREBP-1c) decreased, while the phosphorylated hormone sensitive lipase (p-HSL), the carnitine palmitoyltransferase 1α (CPT1α), and the peroxisome proliferator-activated receptor coactivator-1α (PGC1α) increased, which were responsible for the fatty acid synthesis, lipolysis, and fatty acid β-oxidation, respectively.
Conclusions: Sesamol had a positive effect on anti-obesity and ameliorated the metabolic disorders of obese mice. The possible mechanism of sesamol might be the regulation of lipid metabolism in the liver.
Sesamol is a phenol derivative of sesame oil and a potent anti-oxidant, anti-inflammatory, anti-hepatotoxic, and anti-aging compound. We investigated the effects of sesamol on the molecular mechanisms of adipogenesis in 3T3-L1 preadipocytes. The intracellular lipid accumulation accompanied by increased extracellular release of free glycerol was decreased during differentiation on treating 3T3-L1 with sesamol. Sesamol treatment on 3T3-L1 inhibited adipogenic differentiation by down-regulating adipogenesis-related factors (C/EBPα, PPARγ, and SREBP-1). Lipid accumulation was repressed by decreasing fatty acid synthase and by up-regulating lipolysis-response genes (HSL and LPL).
The molecular mechanisms of sesamol-induced inhibition in adipogenesis were mediated by increased levels of phosphorylated adenosine monophosphate-activated protein kinase and its substrate acetyl-CoA carboxylase. Sesamol treatment, in turn, modulated the different members of the mitogenactivated protein kinase family by suppressing phosphorylation of ERK 1/2 and JNK and by increasing the phosphorylation of p38. In summary, sesamol inhibits adipogenic differentiation by reducing phosphorylation levels of ERK 1/2 and JNK while inducing lipolysis by activating p38 and AMPK. Our results demonstrate that the molecular mechanisms of in vitro anti-obesity effects of sesamol are due to the combined effects of preventing both lipid accumulation and adipogenesis.
Sesamol Alleviates Obesity -Related Hepatic Steatosis via Activating Hepatic PKA Pathway
This study aimed to investigate the effect of sesamol (SEM) on the protein kinase A (PKA) pathway in obesity-related hepatic steatosis treatment by using high-fat diet (HFD)-induced obese mice and a palmitic acid (PA)-treated HepG2 cell line. SEM reduced the body weight gain of obese mice and alleviated related metabolic disorders such as insulin resistance, hyperlipidemia, and systemic inflammation. Furthermore, lipid accumulation in the liver and HepG2 cells was reduced by SEM. SEM downregulated the gene and protein levels of lipogenic regulator factors, and upregulated the gene and protein levels of the regulator factors responsible for lipolysis and fatty acid β-oxidation. Meanwhile, SEM activated AMP-activated protein kinase (AMPK), which might explain the regulatory effect of SEM on fatty acid β-oxidation and lipogenesis.
Results: Microarray analyses showed that components of the WNT/β-catenin pathway including β-catenin, cyclin D1 and dishevelled 2 were up-regulated more than two-fold as a result of SH21B treatment during adipogenesis, which were confirmed by real-time PCR and western blotting. Modulation of the WNT/β-catenin pathway by SH21B resulted in the nuclear accumulation of β-catenin. Both intracellular lipid droplet formation and expressions of adipogenic genes including PPARγ, C/EBPα, FABP4 and LPL, which were inhibited by SH21B, were significantly recovered by β-catenin siRNA transfection.
Conclusions: SH21B modulates components of the WNT/β-catenin pathway during adipogenesis, and β-catenin plays a crucial role in the anti-adipogenic mechanism of SH21B.
Transcriptome Analyses for the Anti-Adipogenic Mechanism of an Herbal Composition
From microarray analyses, we identified 2,568 genes of which expressions were changed more than two-fold by SH21B, and the clustering analyses of these genes resulted in 9 clusters. Three clusters among the 9 showed down-regulation by SH21B (cluster 4, cluster 6 and cluster 9), and two clusters showed up-regulation by SH21B (cluster 7 and cluster 8) during the adipogenesis of 3T3-L1 cells. It was found that many genes related to cell proliferation and adipogenesis were included in these clusters.
Clusters 4, 6 and 9 included genes which were related with adipogenesis induction and cell cycle arrest. Clusters 7 and 8 included genes related to cell proliferation as well as adipogenesis inhibition. These results suggest that the mechanisms of the anti-adipogenic effects of SH21B may be the modulation of genes involved in cell proliferation and adipogenesis.
shikonin (Lithospermun erythrorhizon)
Shikonin-stimulated glucose uptake was not strongly inhibited by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase (PI3K). In vitro phosphorylation analyses revealed that shikonin did not induce tyrosine phosphorylation of the insulin receptor, but significantly induced both Thr-308 and Ser-473 phosphorylation of Akt. Our results suggest that in 3T3-L1 adipocytes, shikonin action is not mediated primarily via the insulin receptor/PI3K pathway, but rather via another distinct tyrosine kinase-dependent pathway leading to glucose uptake involving Akt phosphorylation.
Results: Shikonin effectively suppressed adipogenesis and downregulated the protein levels of 2 major transcription factors, PPARγ and C/EBPα, as well as the adipocyte specific gene aP2 in a dose-dependent manner. qRT-PCR analysis revealed that shikonin inhibited mRNA expression of adipogenesis-related genes, such as PPARγ, C/EBPα, and aP2. Adipocyte differentiation was mediated by ERK 1/2 phosphorylation, which was confirmed by pretreatment with PD98059 (an ERK 1/2 inhibitor) or FGF-2 (an ERK 1/2 activator). The phosphorylation of ERK1/2 during the early stages of adipogenesis in 3T3-L1 cells was inhibited by shikonin. We also confirmed that FGF-2-stimulated ERK 1/2 activity was attenuated by shikonin.
Conclusions: These results demonstrate that shikonin inhibits adipogenic differentiation via suppression of the ERK signaling pathway during the early stages of adipogenesis.
Shikonin Inhibits Fat Accumulation in 3T3‐L1 Adipocytes
The half inhibitory concentration, IC50, for the inhibition of triglyceride accumulation was found to be 1.1 μM. The expression of genes involved in lipid metabolism, such as FABP4 and LPL, were significantly inhibited following shikonin treatment. Shikonin also inhibited the ability of PPARγ and C/EBPα, the major transcription factors of adipogenesis, to bind to their target DNA sequences. The expressions of mRNA and protein of PPARγ and C/EBPa were significantly down‐regulated following shikonin treatment. Among the upstream regulators of adipogenesis, only SREBP1C was found to be down‐regulated by shikonin.
The results of this study suggest that shikonin down‐regulates the expression of SREBP1C and subsequently the expression of PPARγ and C/EBPα. Together, these changes result in the down‐regulation of lipid metabolizing enzymes and reduced fat accumulation.
Shikonin Inhibits Adipogenesis by modulation of the WNT/β-catenin pathway
Significance: This study clearly shows that shikonin inhibits adipogenesis by the modulation of WNT/β-catenin pathway in vitro, and also suggests that WNT/β-catenin pathway can be used as a therapeutic target for obesity and related diseases using a natural compound like shikonin, even though the in vivo effects of shikonin and its clinical significance remain to be elucidated.
Shikonin Inhibits Adipogenic Differentiation via regulation of mir-34a-FKBP1B
Shikonin is a naturally occurring naphthoquinone pigment and a major constituent present in Lithospermum erythrorhizon. Since microRNAs (miRNAs) are one of the key post-transcriptional regulators of adipogenesis, their manipulation represents a potential new strategy to inhibit adipogenesis. Our aim was to investigate shikonin-dependent inhibition of adipogenesis with an emphasis on miRNA-related processes. Mir-34a increased during induced adipogenesis, and this was suppressed in the presence of shikonin. mRNA expression of FKBP1B, a suggested target of mir-34a according to bioinformatics studies, decreased during adipogenesis, but was recovered by shikonin treatment, which reversely correlated with mir-34a expression. A mir-34a inhibitor suppressed MDI-induced adipogenesis by blocking PPARγ and C/EBPα expression, while suppression of mir-34a recovered MDI-induced down-regulation of FKBP1B expression. A mir-34a mimic decreased FKBP1B mRNA expression in 3T3-L1 preadipocytes.
Blood insulin and leptin levels were significantly decreased by shikonin supplementation. Shikonin supplementation reduced protein content and mRNA expression of adipogenesis-related genes in white adipose tissue and lipogenesis-related genes in the liver, along with hepatic lipid content. Moreover, shikonin increased mRNA expression of the β-oxidation genes PPAR-α, PGC-1α, and ACOX1 in liver and skeletal muscle. Shikonin prevents high-fat diet-induced obesity in mice and may be a novel therapeutic approach for obesity prevention by modulating adipogenesis, lipogenesis, and fatty acid oxidation.
Sibiraea angustata
Treatment with SAW also prevented the localization of C/EBPβ to the centromeres. Taken together, our results show that SA has a potent antiadipogenic effect in 3T3-L1 cells due to the inhibition of adipocyte differentiation and adipogenesis. We propose that SA may be used as a safe and effective neutraceutical to manage obesity.
Effects and mechanism of Sibiraea angustata on lipid metabolism in hight-fatted rats
AMPK protein expression in adipose tissue were detected by Western Blot.Compared with the model group, the diameter of adipocytes were reduced while the number increased after adminiseration of Sibiraea angustata for 8 weeks. The levels of adiponectin, adipoR2, AMPK and PPARgamma mRNA were increased siginficantly. The expression of AMPK protein was also up-regulated significantly.Sibiraea angustata has anti-obesity effect. The mechanism may be related to the adiponectin signal transduction pathway.
Silibinin (Milk thistle)

Results: Compared with the other carotenoids evaluated, siphonaxanthin potently Inhibited Adipocyte Differentiation. Siphonaxanthin significantly suppressed lipid accumulation at noncytotoxic concentrations of 2.5 and 5 μmol/L by 29% and 43%, respectively. The effects of siphonaxanthin were largely limited to the early stages of Adipogenesis. Siphonaxanthin significantly Inhibited protein kinase B phosphorylation by 48% and 72% at 90 and 120 min, respectively. The expressions of key Adipogenesis genes, including CCAAT/enhancer binding protein α (Cebpa), peroxisome proliferator activated receptor γ (Pparg), fatty acid binding protein 4 (Fabp4), and stearoyl coenzyme A desaturase 1 (Scd1), were elevated by 1.6- to 166-fold during Adipogenesis. After 8 d of Adipocyte Differentiation, siphonaxanthin significantly lowered gene expression of Cebpa, Pparg, Fabp4, and Scd1 by 94%, 83%, 95%, and 90%, respectively. Moreover, oral administration of siphonaxanthin to KK-Ay mice significantly Reduced the total weight of white Adipose Tissue (WAT) by 13%, especially the mesenteric WAT by 28%. Furthermore, siphonaxanthin administration Reduced lipogenesis and enhanced fatty acid oxidation in Adipose Tissue. Siphonaxanthin was observed to highly accumulate in mesenteric WAT, and the accumulation in the mesenteric WAT was almost 2- and 3-fold that in epididymal (P = 0.14) and perirenal (P < 0.05) WAT, respectively.
Conclusion: These results provide evidence that siphonaxanthin may effectively regulate Adipogenesis in 3T3-L1 cells and Diabetic KK-Ay mice.
Soyasaponin Ab (field‐grown soybean)
Soyasaponin Ab alleviates postmenopausal Obesity through browning of white adipose tissue
Moreover, SA significantly decreased weight gain, adipocyte area, serum metabolic profiles in OVX mice. SA also inhibited the lipid metabolism-related factors but increased the thermoregulatory factors in both in vitro and in vivo. SA treatment further increased energy expenditure through beige fat activation. Overall, we found that SA effectively accelerated energy consumption by stimulating beige fat activation, even under estrogen-deficient conditions. Thus, SA treatment may be a promising strategy for the prevention of postmenopausal obesity by promoting weight loss, reducing fat accumulation, and improving obesity-related metabolic disorders.
Saponins are a diverse group of biologically functional products in plants. Soyasaponins are usually glycosylated, which give rise to a wide diversity of structures and functions. In this study, we investigated the effects and molecular mechanism of soyasaponins Aa and Ab in regulating adipocyte differentiation and expression of adipogenic marker genes in 3T3-L1 adipocytes. Soyasaponins Aa and Ab dose-dependently inhibited the accumulation of lipids and the expression of adiponectin, adipocyte determination and differentiation factor 1/sterol regulatory element binding protein 1c, adipocyte fatty acid-binding protein 2, fatty acid synthase, and resistin in 3T3-L1 adipocytes.
stearidonic acid (flaxseed)
These results indicate that SDA consumption will be expected to confer the health benefits associated with the consumption of EPA, but not DHA. While the exact molecular mechanisms by which SDA decreases gene transcription is not apparent from this study, the downstream biosynthesis of lipid-derived eicosanoids via its conversion to EPA may be a potential mechanism through which gene transcription effects could be modulated [3T3-L1 Adipocytes. Prostaglandins Other Lipid Mediat. Inhibits Adipogenesis via an autocrine-mediated interleukin-11/glycoprotein 130/STAT1-dependent signaling cascade. J Cell Biochem. Inhibition of 3T3-L1 Preadipocyte Differentiation by prostaglandin F2alpha. Endocrinology.
This study has demonstrated that SDA was able to Inhibit Adipocyte Differentiation and Reduce lipid accumulation in 3T3-L1 Adipocytes through down-regulation of Adipogenic transcription factors and lipid accumulation genes (Fig. 7). These findings warrant further investigation that will develop SDA as a natural and effective agent for the prevention or treatment of Obesity.
Sulforaphene (Raphanus sativus)
Sulforaphene Inhibition of Adipogenesis via Hedgehog Signaling in 3T3-L1 Adipocytes
Obesity is a risk factor for numerous metabolic disorders. In this study, we investigated the effects of the isothiocyanates sulforaphane (SA) and sulforaphene (SE) on Adipogenesis in 3T3-L1 Adipocytes . SE, a compound that is abundant in radish, Inhibited Adipogenesis by suppressing the Adipogenic transcription factors peroxisome proliferator-activated receptor γ (PPARγ , 69.2 ± 2.4%, P < 0.05) and CCAAT/enhancer-binding protein α (C/EBPα , 36.1 ± 3.1%, P < 0.05), thereby reducing Fat Accumulation in 3T3-L1 Adipocytes (45.6 ± 2.7%, P < 0.05); SA was less effective. SE exerted these activities through the activation of the Hedgehog (Hh) signaling pathway by restoring Smo ((2.1 ± 0.2)-fold, P < 0.05) and Gli1 ((2.8 ± 0.1)-fold, P < 0.05) expression, which was suppressed by Adipogenic signals.
These effects of SE were abrogated by treatment with the Hh Inhibit or vismodegib. Thus, SE Inhibits Adipocyte Differentiation via Hh signaling and may be an effective natural agent for preventing Adipocyte hyperplasia and Obesity.
Stinging nettles (Utrica dioica)
Twenty‐two plant extracts out of 133 were found to increase insulin‐stimulated glucose uptake and 18 extracts were found to activate PPARγ, 3 to activate PPARα and γ, 6 to activate PPARδ and γ, and 9 to activate PPARγ, α and δ. Among the 24 different plant species tested in the platform, 50% were shown to contain compounds capable of activating PPARγ and stimulating insulin‐dependent glucose uptake with no or little effect on Adipocyte Differentiation warranting further studies and characterization.
Styrax japonica
Anti-Adipogenic effects in 3T3-L1 cells of acetone extracts and fractions from Styrax japonica fruit
Reduction in reactive oxygen species (ROS) production facilitated by Styrax japonica (SJ) extracts and fractions for Inhibition of Obesity development through Inhibition of Adipogenesis was investigated. Treatment of cells with SJ extracts and fractions did not result in changes in cell viability. SJ extracts and fractions Inhibited lipid accumulation in a dose-dependent manner during Adipogenesis. Expressions of PPARγ and C/EBPα, key Adipogenic markers, were decreased in cells treated with SJ extracts and fractions.
Inhibitory effects of SJ extracts and fractions were the most powerful in the early stages of Differentiation (days 0-2). Intracellular ROS production was decreased in cells treated with SJ extracts and fractions. SJ extracts and fractions can Inhibit Adipogenesis via Reduced ROS levels, which involves down-regulation of Adipogenic transcription factors.
Sulfuretin (Rhus verniciflua)
Exploration of underlying mechanism of anti-Adipogenic activity of sulfuretin
Sulfuretin is a natural flavonoid found in the plant Rhus verniciflua STOKES. The plant has been traditionally used as medicinal agent for antiviral, cathartic, diaphoretic, anti-rheumatic and sedative activities in East Asia. In this study we isolated and identified sulfuretin from R. verniciflua and investigated its anti-adipogenic activity against 3T3-L1 preadipocytes cells. We evaluated the effects of sulfuretin on the adipogenic transcription factors like peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer-binding protein α (C/EBPα), fatty acid synthase (FAS), Fabp4, adiponectin and zinc fingerprint protein (Zfp) 521 by gene expression (real-time QPCR) and Western blot analysis. Sulfuretin treatment at Day 0 and 2 showed significant reduction of lipid production in 3T3-L1 cells in concentration dependent manner. Gene expression analysis (real-time PCR) revealed that sulfuretin inhibited the both major adipogenic factors (C/EBPα, C/EBPβ and PPARγ) and minor adipogenic factors (sterol regulatory element-binding protein (SREBP1c), adiponectin, FAS, Fabp4, Zfp423, and Ebf1). Western blot analysis showed the increased expression of β-catenin and suppression of PPARγ after sulfuretin treatment. Overall, sulfuretin is a natural flavonoid having potent anti-adipogenic activity through the suppression of major adipogenic factors C/EBPα, C/EBPβ and PPARγ, which initiate adipogenesis.
Sulfuretin Prevents Obesity and Metabolic Diseases in Diet Induced Obese Mice
To elucidate the molecular mechanism of sulfuretin in adipocytes, we performed microarray analysis and identified activating transcription factor 3 (Atf3) as a sulfuretin-responsive gene. Sulfuretin elevated Atf3 mRNA and protein levels in white adipose tissue and adipocytes. Consistently, deficiency of Atf3 promoted lipid accumulation and the expression of adipocyte markers. Sulfuretin’s but not resveratrol’s anti-adipogenic effects were diminished in Atf3 deficient cells, indicating that Atf3 is an essential factor in the effects of sulfuretin. These results highlight the usefulness of sulfuretin as a new anti-obesity intervention for the prevention of obesity and its associated metabolic diseases.

A Inhibited the expression of key genes for Adipogenesis. Differences between samples with or without TA in all of the quantitative assays were significant (P < 0.05). These results suggest that TA may be useful for the prevention and treatment of T2D and its associated Obesity. TA may have the potential to become the lead compound in the development of new types of AntiDiabetic pharmaceuticals that are able to Reduce blood glucose levels without increasing Adiposity.

In this in vitro study, we have investigated the ability of Taraxacum officinale (dandelion) to Inhibit Adipocyte Differentiation and lipogenesis in 3T3‐L1 preadipocytes. HPLC analysis of the three plant extracts used in this study—leaf and root extracts and a commercial root powder—identified caffeic and chlorogenic acids as the main phenolic constituents. Oil Red O staining and triglyceride levels analysis showed decreased lipid and triglyceride accumulation, respectively. Cytotoxicity was assessed with the MTT assay showing non‐toxic effect among the concentrations tested.
DNA microarray analysis showed that the extracts regulated the expression of a number of genes and long non‐coding RNAs that play a major role in the control of Adipogenesis. Taken together, our results indicate that the dandelion extracts used in this study may play a significant role during Adipogenesis and lipid metabolism, and thus, supporting their therapeutic interest as potential candidates for the treatment of Obesity.

γ-tocotrienol suppressed the insulin-induced aP2 and C/EBPα mRNA expression, triglyceride accumulation, and PPARγ protein levels compared with insulin. The current results also revealed that γ-tocotrienol Inhibited the insulin-stimulated phosphorylation of Akt but not extracellular signal-regulated kinase (ERK)1/2 in the insulin signaling pathway of 3T3-L1 preadipocytes . Thus, the AntiAdipogenic effect of TRF depends on α-tocotrienol and γ-tocotrienol, and γ-tocotrienol may be a more potent Inhibit or of Adipogenesis than α-tocotrienol.
Therefore, the results of this study suggest that tocotrienol Suppresses insulin-induced Differentiation and Akt phosphorylation in 3T3-L1 preadipocytes . Furthermore, tocotrienol could act as an AntiAdipogenic vitamin in the nutrient-mediated regulation of body fat through its effects on Differentiation.

Anti-Adipogenic effect of oat hull extract containing tricin on 3T3-L1 Adipocytes
Oat hull ethyl acetate extract (50 μg/μL) significantly Inhibited triglyceride accumulation in 3T3-L1 Adipocytes without causing cytotoxicity. The major active compound in the extract was isolated and identified as 5,7,4′-trihydroxy-3′,5′-dimethoxyflavone, tricin by Mass spectrometry and 1H and 13C NMR analyses. The tricin content in oat hull was approximately 18 mg/kg dry weight, and tricin constituted 9.6% of the total phenolic compounds in oat hull extract. The treatment of 3T3-L1 cells either with extract or purified tricin resulted in decreased lipid accumulation. The extract significantly decreased the activity of glycerol-3-phosphate dyhydrogenase, a marker of Adipocyte Differentiation in a dose dependent manner.
Trigonella foenum
Results: We found that INDUS810 can reduce high-fat diet-induced weight increase in epididymal white adipose tissue, interscapular brown adipose tissue and liver, as well as serum levels of total cholesterol and low-density lipoprotein cholesterol. Moreover, the insulin sensitivity was significantly improved in INDUS810-treated obese mice. In 3T3-L1 adipocytes, we found that INDUS810 could inhibit lipid accumulation at either differentiating or mature stages, and increase lipolysis activity in mature adipocytes. Additionally, INDUS810 has no effects on cell viability nor the expressions of adipocyte differentiation markers like fatty acid synthase, peroxisome proliferator-activated receptor γ and CCAAT/enhancer-binding protein α. In contrast, INDUS810 can increase protein levels of peroxisome proliferator-activated receptor α, peroxisome-proliferator-activated receptor-γ co-activator 1β, sirtuin 1 and sirtuin 3. Of note, INDUS810 can activate adenosine monophosphate-activated protein kinase, which leads to the reduction of lipid contents in adipocytes.
Conclusion: Our in vitro and in vivo studies suggest that INDUS810 is a potential anti-obesity agent, and this action depends on activate adenosine monophosphate-activated protein kinase activation.
In addition, polyphenol stilbenes improved the uptake of 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) by promoting the phosphorylation of protein kinase B (AKT) and AMP-activated protein kinase (AMPK). In present studies, it was found that polyphenol stilbenes had the ability to scavenge reactive oxygen species (ROS).
Results from adenosine triphosphate (ATP) production and mitochondrial membrane potentials suggested that mitochondria play a critical role in insulin resistance and related signaling activation, such as AKT and AMPK. Rhaponticin, one of the stilbenes from fenugreek, had the strongest activity among the three compounds in vitro. Future studies will focus on mitochondrial biogenesis and function.

Taken together, these results revealed that the Flavonoids from TA possessed an Inhibitory effect on Adipogenesis through downregulation of Adipogenic transcription factors and genes associated with lipid metabolism, and the upregulation of insig 1 and 2, suggesting that the Flavonoids from TA may be potential therapeutic agents for the prevention and treatment of Obesity.
Ulmus pumila
Obesity and its associated metabolic disorders has become a major obstacle in improving the average life span. In this regard therapeutic approach using natural compounds are currently receiving much attention. Herbal compounds rich in triterpenes are well known to regulate glucose and lipid metabolism. Here, we have found that Ulmus pumila (UP) contained at least four different triterpenoids and Inhibited Adipogenesis of 3T3-L1 cells.
Ursolic Acid
Ursolic acid Inhibits Adipogenesis in 3T3-L1 Adipocytes through LKB1/AMPK pathway
Methods and results: The 3T3-L1 preadipocytes were induced to differentiate in the presence or absence of UA for 6 days. The cells were determined for proliferation, differentiation, fat accumulation as well as the protein expressions of molecular targets that regulate or are involved in fatty acid synthesis and oxidation. The results demonstrated that ursolic acid at concentrations ranging from 2.5 µM to 10 µM dose-dependently attenuated adipogenesis, accompanied by reduced protein expression of CCAAT element binding protein β (C/EBPβ), peroxisome proliferator-activated receptor γ (PPARγ), CCAAT element binding protein α (C/EBPα) and sterol regulatory element binding protein 1c (SREBP-1c), respectively. Ursolic acid increased the phosphorylation of acetyl-CoA carboxylase (ACC) and protein expression of carnitine palmitoyltransferase 1 (CPT1), but decreased protein expression of fatty acid synthase (FAS) and fatty acid-binding protein 4 (FABP4). Ursolic acid increased the phosphorylation of AMP-activated protein kinase (AMPK) and protein expression of (silent mating type information regulation 2, homolog) 1 (Sirt1). Further studies demonstrated that the anti-adipogenic effect of UA was reversed by the AMPK siRNA, but not by the Sirt1 inhibitor nicotinamide. Liver kinase B1 (LKB1), the upstream kinase of AMPK, was upregulated by UA. When LKB1 was silenced with siRNA or the inhibitor radicicol, the effect of UA on AMPK activation was diminished.
Ursolic acid, a promising dietary bioactive compound of anti‐Obesity (1045.40)
The anti-adipogenic effect of UA can be reversed by AMPK siRNA but not Sirt1 inhibitor. Moreover, when LKB1 is silenced or inhibited, the effect of UA on AMPK activation diminishes. These results demonstrate that ursolic acid inhibits preadipocyte differentiation and adipogene.

Vanillic Acid (Vanilla planifolia)
Veratrum nigrum
Veratri nigri rhizoma et radix (Veratrum nigrum L.) and its constituent jervine Prevent Adipogenesis

Viburnum opulus L.
Thirty-two phenolic compounds with chlorogenic acid as the dominant compound were identified in PJ which revealed significant biological activity. These data contribute to elucidate V. opulus juice phenolic compounds’ molecular mechanism in Adipogenesis regulation in 3T3-L1 cells and dietary fat Lipolysis.
Vitexilactone (Vitex trifolia)
Vitexin, orientin and other Flavonoids from Spirodela polyrhiza Inhibit Adipogenesis in 3T3‐L1 cells
Vitisin (Iris lactea Pall)
Vitisin A Inhibits Adipocyte Differentiation through cell cycle arrest in 3T3-L1 cells
Inhibition of Adipocyte Differentiation is one approach among the Anti-Obesity strategies. This study demonstrates that vitisin A, a resveratrol tetramer, Inhibits Adipocyte Differentiation most effectively of 18 stilbenes tested. Fat Accumulation and PPARγ expression were decreased by vitisin A in a dose-dependent manner. Vitisin A significantly Inhibited Preadipocyte proliferation and consequent Differentiation within the first 2 days of treatment, indicating that the Anti-Adipogenic effect of vitisin A was derived from anti-proliferation. Based on cell cycle analysis, vitisin A blocked the cell cycle at the G1-S phase transition, causing cells to remain in the Preadipocyte state. Vitisin A increased p21 expression, while the Rb phosphorylation level was Reduced. Therefore, vitisin A seems to induce G1 arrestthrough p21- and consequent Rb-dependent suppression of transcription. On the other hand, ERK and Akt signaling pathways were not involved in the anti-mitotic regulation by vitisin A. Taken together, these results suggest that vitisin A Inhibits Adipocyte Differentiation through Preadipocyte cell cycle arrest.
Wasabi (Wasabia japonica)
Western blot analysis results showed that the protein expression levels of both PPARγ and C/EBPα were also Inhibited by WLE. Thus, WLE suppressed the Differentiation of 3T3-L1 preadipocytes, and the suppressive effect was mediated, in part, through the altered regulation of PPARγ, C/EBPα, and other specific genes, such as aP2. These results suggest that WLE may Prevent Obesity and insulin resistance by Inhibiting the Differentiation of preadipocytes.
Wedelolactone (Wedelia calendulacea)

Furthermore, the Antioxidants ascorbic acid and 2-mercaptoethanol prevented XN and IXN-induced ROS generation and apoptosis. Immunoblotting analysis showed an increase in the levels of cytoplasmic cytochrome c and cleaved poly (ADP-ribose) polymerase (PARP) by XN and IXN. Concomitantly, we observed activation of the effectors caspase-3/7. In maturing preadipocytes both XN and IXN were effective in reducing lipid content, XN being more potent. Moreover, the major Adipocyte marker proteins such as PPARγ, C/EBPα, and aP2 decreased after treatment with XN during the maturation period and that of DGAT1 decreased after treatment with XN and IXN. Taken together, our data indicate that both XN and IXN Inhibit Differentiation of preadipocytes , and induce apoptosis in mature Adipocytes, but XN is more potent.
zeaxanthin (MARIGOLD genus tagetes)
Anti–Obesity effects of zeaxanthin on 3T3-L1 Preadipocyte and high fat induced obese mice
Zeaxanthin, a type of carotenoid, has been proven to exhibit anti-lipogenesis effect; however, the detailed mechanism of this effect is less known. Herein, we evaluated the effects of zeaxanthin on the inhibition of adipogenesis in 3T3-L1 adipocytes and obesity in high-fat diet fed C57BL/6J mice. Zeaxanthin significantly decreased the intracellular lipid content in a dose-dependent manner (5–15 μM) in adipocytes without causing cytotoxicity. In high-fat-diet-induced obese mice, oral administration of 20 mg kg−1 zeaxanthin attenuated the progression of obesity and improved dyslipidemia. It exhibits an anti-adipogenic effect via down-regulating the transcriptional factors and adipocyte-specific genes involved in adipogenesis, both in vitro and in vivo.
Zeaxanthin promotes mitochondrial biogenesis and Adipocyte browning via AMPKα1 activation
Zeaxanthin (ZEA), a type of oxygenated carotenoid with strong antioxidant activity, has previously been found to exhibit an anti-lipogenesis effect. In the present study, we investigated the effect of ZEA on brown-like adipocyte formation and mitochondrial biogenesis in 3T3-L1 adipocytes. Brown adipocyte-specific markers, mitochondrial biogenesis and oxidative stress, and the involvement of AMP-activated protein kinase (AMPK) α1 were assessed. ZEA treated adipocytes demonstrated a brown-like pattern, with upregulated expression of uncoupling protein 1 (UCP1) and other brown adipocyte markers. In addition, ZEA intervention induced a dramatic increase in mitochondrial DNA (mtDNA) content and in the mRNA levels of genes associated with mitochondrial biogenesis.
Zizyphus jujube
Effect of Zizyphus jujuba Extract on the Inhibition of Adipogenesis in 3T3-L1 preadipocytes
Obesity has been often associated with the occurrence of cardiovascular diseases, type 2 Diabetes, and cancer. The development of Obesity is also accompanied by significant Differentiation of preadipocytes into Adipocytes. In this study, we investigated the activity of α-mangostin, a major xanthone component isolated from the stem bark of G. malaccensis, on glucose uptake and Adipocyte Differentiation of 3T3-L1 cells focusing on PPARγ, GLUT4, and leptin expressions. α-Mangostin was found to Inhibit cytoplasmic lipid accumulation and Adipogenic Differentiation.
Acorus calamus (Araceae) has been incorporated into traditional medicines and used in food supplements for hundreds of years. The aim of this study was to investigate the Inhibitory effects of A. calamus essential oil (calamus oil) on the Differentiation of 3T3-L1 preadipocytes into Adipocytes. The major Anti-Adipogenic component of calamus oil was purified and identified as β-asarone. β-Asarone significantly Inhibited intracellular lipid accumulation during Adipocyte Differentiation in a concentration-dependent manner.
In addition, the protein and mRNA expression levels of C/EBPβ, C/EBPα, and PPARγ were decreased in 3T3-L1 cells treated with β-asarone during Differentiation. Phosphorylation of ERK1/2, which is known to regulate the early phase of Adipogenesis, was attenuated by β-asarone treatment. These results suggest that β-asarone exerts Anti-Adipogenic activity, in part by suppressing the expression of Adipogenic transcription factors.

β-Cryptoxanthin Suppresses the Adipogenesis of 3T3-L1 cells via RAR Activation
We recently reported that the oral intake of β-cryptoxanthin exerted Anti-Obesity effects by lowering visceral fat levels. In the present study, we characterized the molecular mechanisms underlying the lipid-lowering effects of β-cryptoxanthin on 3T3-L1 cells. Consistent with our previous findings, β-cryptoxanthin rapidly Reduced the level of intracellular lipids in 3T3-L1 cells as assessed by Oil red O staining. Using an in vitro nuclear receptor binding assay, we demonstrated the ability of β-cryptoxanthin to bind to and activate members of the retinoic acid receptor (RAR) family. Accordingly, treatment of cells with LE540, an RAR antagonist, abolished the β-cryptoxanthin-dependent suppression of 3T3-L1 Adipogenesis, suggesting that β-cryptoxanthin mediates its effects on 3T3-L1 cells via RAR activation.
13-Methylberberine (Coptis chinensis)
Lipid metabolism modulation is a main focus of Metabolic Syndrome research, an area in which many natural and synthetic chemicals are constantly being screened for in vitro and in vivo activity. Berberine, a benzylisoquinoline plant alkaloid, has been extensively investigated for its Anti-Obesity effects and as a potential cholesterol and triglyceride-lowering drug. We screened 11 protoberberine and 2 benzophenanthridine alkaloids for their Anti-Adipogenic effects on 3T3-L1 Adipocytes and found that 13-methylberberine exhibited the most potent activity. 13-Methylberberine down-regulated the expression of the main Adipocyte Differentiation transcription factors, peroxisome proliferator-activated receptor gamma (PPARγ ) and CCAAT enhancer binding protein alpha (C/EBPα), as well as their target genes. PPARγ, C/EBPα, and sterol regulatory element binding protein 1 (SREBP-1) protein levels were Reduced, and this lipid-reducing effect was attenuated by an AMP-activated protein kinase (AMPK) Inhibit or, indicating that the effect of this compound requires the AMPK signaling pathway. Decreased Akt phosphorylation suggested Reduced de novo lipid synthesis. C-13 methyl substitution of berberine increased its accumulation in treated cells, suggesting that 13-methylberberine has improved absorption and higher accumulation compared to berberine. Our findings suggest that 13-methylberberine has potential as an Anti-Obesity drug.
18β-Glycyrrhetinic acid (Liquorice)
18β-Glycyrrhetinic acid (18β-GA) obtained from the herb liquorice has various pharmacological properties including anti-inflammatory and anti-bacterial activities. However, potential biological Anti-Obesity activities are unclear. In this study, novel biological activities of 18β-GA in the Adipogenesis of 3T3-L1 preAdipocyte s and in Lipolysis of differentiated Adipocytes were identified. Mouse 3T3-L1 cells were used as an in vitro model of Adipogenesis and Lipolysis, using a mixture of insulin/dexamethasone/3-isobutyl-1-methylxanthine (IBMX) to induce Differentiation. The amount of lipid droplet accumulation was determined by an AdipoRed assay. The expression of several Adipogenic transcription factors and enzymes was investigated using real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blotting. 18β-GA dose-dependently (1–40 μM) significantly decreased lipid accumulation in maturing preadipocytes. In 3T3-L1 preadipocytes, 10 μM of 18β-GA down-regulated the transcriptional levels of the peroxisome proliferator-activated receptor γ, CCAAT/enhancer-binding protein α and adiponectin, which are markers of Adipogenic Differentiation via Akt phosphorylation. Also, in differentiated Adipocytes, 18β-GA increased the level of glycerol release and up-regulated the mRNA of hormone-sensitive lipase, adipose TG lipase and perilipin, as well as the phosphorylation of hormone-sensitive lipase at Serine 563. The results indicate that 18β-GA alters fat Mass by directly affecting Adipogenesis in maturing preadipocytes and Lipolysis in matured Adipocytes. Thus, 18β-GA may be useful for the treatment of Obesity.
Methods and results: 18β‐glycyrrhetinic acid (18β‐GA) alleviated the effects of CB 1R agonist, anandamide (AEA ) on CB 1R signaling in a concentration‐dependent manner. Consistently, 18β‐GA suppressed AEA ‐induced Adipocyte Differentiation in 3T 3‐L 1 cells through the downregulation of AEA ‐induced MAPK activation and expression of Adipogenic genes including C /EBP ‐α and PPAR‐γ. The protein levels of fatty acid synthase and stearoyl‐C oA desaturase 1 were also decreased and the phosphorylation of acetyl‐CoA carboxylase was increased in 18β‐GA pretreated cells. The supplementation of 18β‐GA significantly lowered body weight, fat weight, and plasma lipids levels in obese animal models.
Conclusion: These results may provide a novel insight into the molecular mechanism involved in anti‐Adipogenic and anti‐Obesity effects of 18β‐GA by suppressing the activation of CB 1R induced by AEA . Thus, 18β‐GA may exert beneficial effects against Obesity‐related metabolic disorders.
2,6-Dimethoxy-1,4-benzoquinone (vanilla planifolia)
2,6-Dimethoxy-1,4-benzoquinone is a natural phytochemical present in fermented wheat germ. It has been reported to ex- hibit anti-inflammatory, antitumor, and antibacterial activ- ities. However, the Anti-Adipogenic effects of 2,6-dimethoxy- 1,4-benzoquinone and the mechanisms responsible have not previously been elucidated. Such findings may have ramifica- tions for the treatment of Obesity.
2,6-Dimethoxy-1,4-benzo- quinone (5 and 7.5 μM) significantly Reduced the expression of various Adipogenic transcription factors, including peroxisome proliferator-activated receptor-γ and CCAAT/enhancer bind- ing protein α as well as Adipocyte protein 2 and fatty acid syn- thase. 2,6-Dimethoxy-1,4-benzoquinone upregulated AMP- dependent protein kinase phosphorylation and Inhibited the mature form of sterol regulatory element-binding protein 1c. Notably, 2,6-dimethoxy-1,4-benzoquinone attenuated mam- malian target of rapamycin complex 1 activity in 3T3-L1 and mouse embryonic fibroblast cells.
These findings highlight a potential role for 2,6-dimethoxy-1,4-benzoquinone in the suppression of Adipogenesis. Further studies to determine the Anti-Obesity effects of 2,6-dimethoxy-1,4-benzoquinone in animal models appear warranted.
Benzoic acid (bilberry)
Obesity is a serious health issue in many industrialized countries. It is a medical condition with excessive levels of fat accumulated in Adipocytes. The objective of the present study was to determine the Inhibitory effect of 3-chloro-4,5-dihydroxybenzaldehyde (CDB) on Adipogenesis in 3T3-L1 Adipocyte cells. CDB suppressed the Differentiation and decreased lipid accumulation and triglyceridescontents in 3T3-L1 Adipocytes. Its suppression effect on Fat Accumulation was mediated via expression of Adipogenesis factors (C/EBPα , SREBP-1c, PPARγ, and adiponectin) during Adipocyte Differentiation in white Adipocyte cells. CDB’s ability to supfpress Fat Accumulation was increased in a concentration-dependent manner.
It Inhibited fatty acid synthesis related proteins including FAS, FABP4, leptin, and perilipin. It also increased expression of phosphorylated AMPK in Adipocytes cells. These observations suggest that CDB has potential Anti-Obesity effect with ability to improve metabolic diseases.
3,5-Dicaffeoyl-Epi-Quinic Acid (Halophyte Atriplex gmelinii)
Atriplex gmelinii is an edible halophyte that has been suggested to possess various health benefits. In the present study, 3,5-dicaffeoyl-epi-quinic acid (DEQA) isolated from A. gmelinii was tested for its ability to Prevent Adipogenesis in 3T3-L1 cells. Also, the molecular mechanisms by which DEQA affects Differentiation of 3T3-L1 cells were investigated. The introduction of DEQA to differentiating 3T3-L1 preAdipocytes resulted in suppressed Adipogenesis and lowered expression of Adipogenesis-related factors, PPARγ, C/EBPα, and SREBP-1c. Treatment of 3T3-L1 Adipocytes with DEQA notably decreased the levels of phosphorylated p38, ERK, and JNK.
In addition, presence of DEQA upregulated the levels of both inactive and phosphorylated adenosine monophosphate-activated protein kinase (AMPK) and its substrate, acetyl-CoA carboxylase (ACC). Taken together, current results indicated that DEQA exhibited a significant AntiAdipogenesis activity by activation of AMPK and downregulation of MAPK signal pathways in 3T3-L1 preAdipocytes.
3,5,6,7,8,3′,4′-heptamethoxyflavone (Tangerine peel)
Adipogenesis by 5-Hydroxy-3,6,7,8,3′,4′-Hexamethoxyflavone from Orange Peel in 3T3-L1 cells Suppression of Adipogenesis by 5-Hydroxy-3,6,7,8,3′,4′-Hexamethoxyflavone from Orange Peel in 3T3-L1 cells
tthe Inhibition rate of lipid accumulation was compared between 5-OH-HxMF and 3,5,6,7,8,3′,4′-heptamethoxyflavone (HpMF). 5-OH-HxMF Inhibited lipid accumulation 15–20% more than HpMF did, indicating that hydroxyl group at position 5 can be a key factor in the suppression of Adipogenesis.
Heptamethoxyflavone Inhibits Adipogenesis via enhancing PKA signaling
Furthermore, the induction of phosphorylation of AMPK and ACC by HMF was abolished by RNA interference targeting PKACα. Taken together, our results suggest that HMF might Inhibit the early stage of Adipogenesis via the activation of PKA signaling in 3T3-L1 cells.
Excess energy is a major cause of Obesity and it can increase the number and size of Adipocytes, eventually expanding the Adipose Tissue. Adipose Tissue can be deposited in the intra-abdominal area and it affects the function of other organs, such as the liver, pancreas, and skeletal muscle, therefore, it is a risk factor related to liver disease and type 2 Diabetes. Diet modification and the Inhibition of the cause of Obesity-related molecular mechanisms could be solutions for controlling the expansion of adipose Mass and decreasing the prevalence of Obesity . Polymethoxyflavones (PMFs) and hydroxylated polymethoxyflavones (HPMFs) such as nobiletin, tangeretin, 5-demethyltangeretin, and 5-demethylnobiletin are unique Flavonoids that almost exclusively exist in the peel of the citrus genus and have many health-beneficial effects. However, their lipophilic structure and characteristics give them poor aqueous solubility and low oral bioavailability.
Prevention of Obesity and Hyperlipidemia by Heptamethoxyflavone in High-fat Diet-induced Rats
Polymethoxyflavones (PMFs) have been shown to Prevent Obesity, ameliorate type 2 Diabetes, and regulate lipid metabolism in vitro and in vivo. However, little is known about the contribution of 3,5,6,7,8,3′,4′-heptamethoxyflavone (HMF) to Prevent Obesity and regulate lipid metabolism in vivo. We aimed to investigate the potential efficacy of HMF on preventing Obesity and hyperlipidemia in rats fed a high-fat diet (HFD) and its underlying mechanisms. Male Sprague–Dawley rats were fed a normal diet or an HFD with or without HMF (0.02%, 0.04% and 0.08%, w/w) for 6 weeks. The supplementation of HMF not only significantly decreased body Weight Gain (HFD, 336.50 ± 18.84 g; LHMF, 309.43 ± 20.74 g; MHMF, 296.83 ± 13.88 g; HHMF, 265.71 ± 19.09 g; respectively, p< 0.05) and Adipose Tissues weight (p < 0.05), but also markedly lowered serum levels of total cholesterol, triacylglycerol, and low-density lipoprotein cholesterol (p < 0.05) in the sixth week in a dose-dependent manner compared with the HFD group.
6-Gingerol (Ginger)
6-Gingerol prevents Adipogenesis and the accumulation of cytoplasmic lipid droplets in 3T3-L1 cells
6-gingerol diminished the insulin-stimulated serinephosphorylation of Akt (Ser473) and GSK3β (Ser9). These results suggest that 6-gingerol effectively Suppresses Adipogenesis and that it exerts its role mainly through the significant down-regulation of PPARγ and C/EBPα and subsequently Inhibits FAS and aP2 expression. 6-Gingerol also Inhibited Differentiation in 3T3-L1 cells by attenuating the Akt/GSK3β pathway. Our findings provide important insights into the mechanisms underlying the Anti-Adipogenic activity of 6-gingerol.
Genistein 6,7,4′-Tri hydroxy isoflavone
Conclusion: Our results suggest that 6,7,4′‐THIF Suppresses Adipogenesis in 3T3‐L1 preadipocytes by directly targeting PI3K. Soy isoflavones like 6,7,4′‐THIF may have potential for development into novel treatment strategies for chronic Obesity.
Warnings: As with any supplement, consult your medical practitioner prior to using this product if you are pregnant, may become pregnant, breastfeeding, taking medication, other dietary supplements or have a medical condition. These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. *Daily Value has not been established.
| Size | 25g, 50g, 100g, 200g, 300g |
|---|






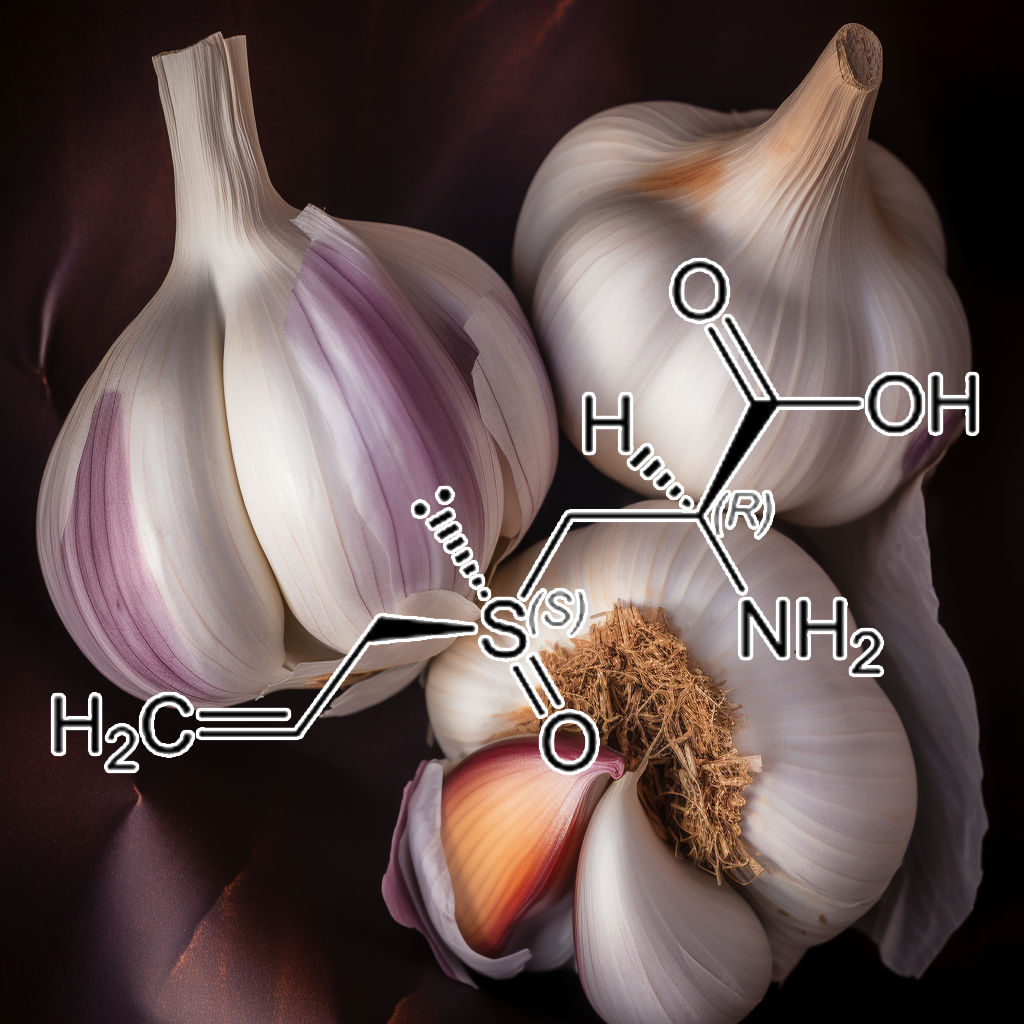















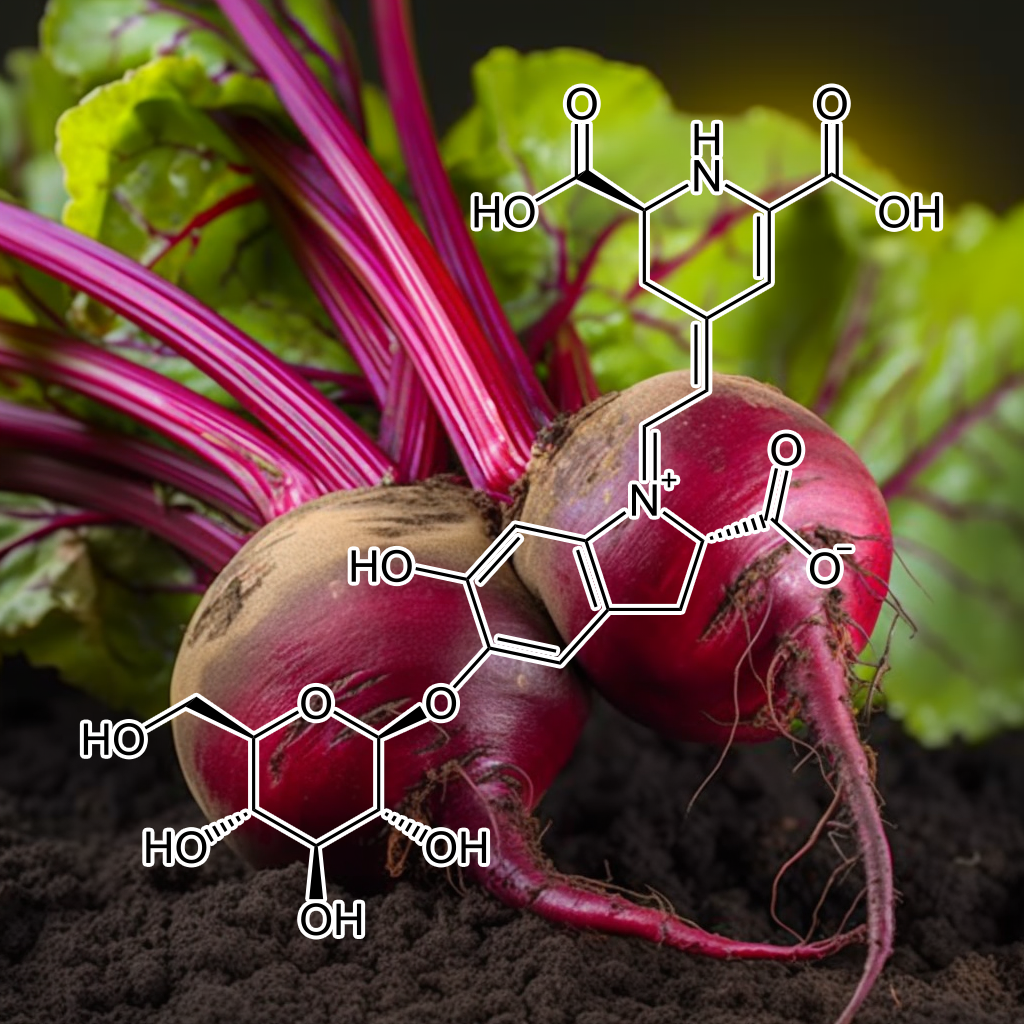




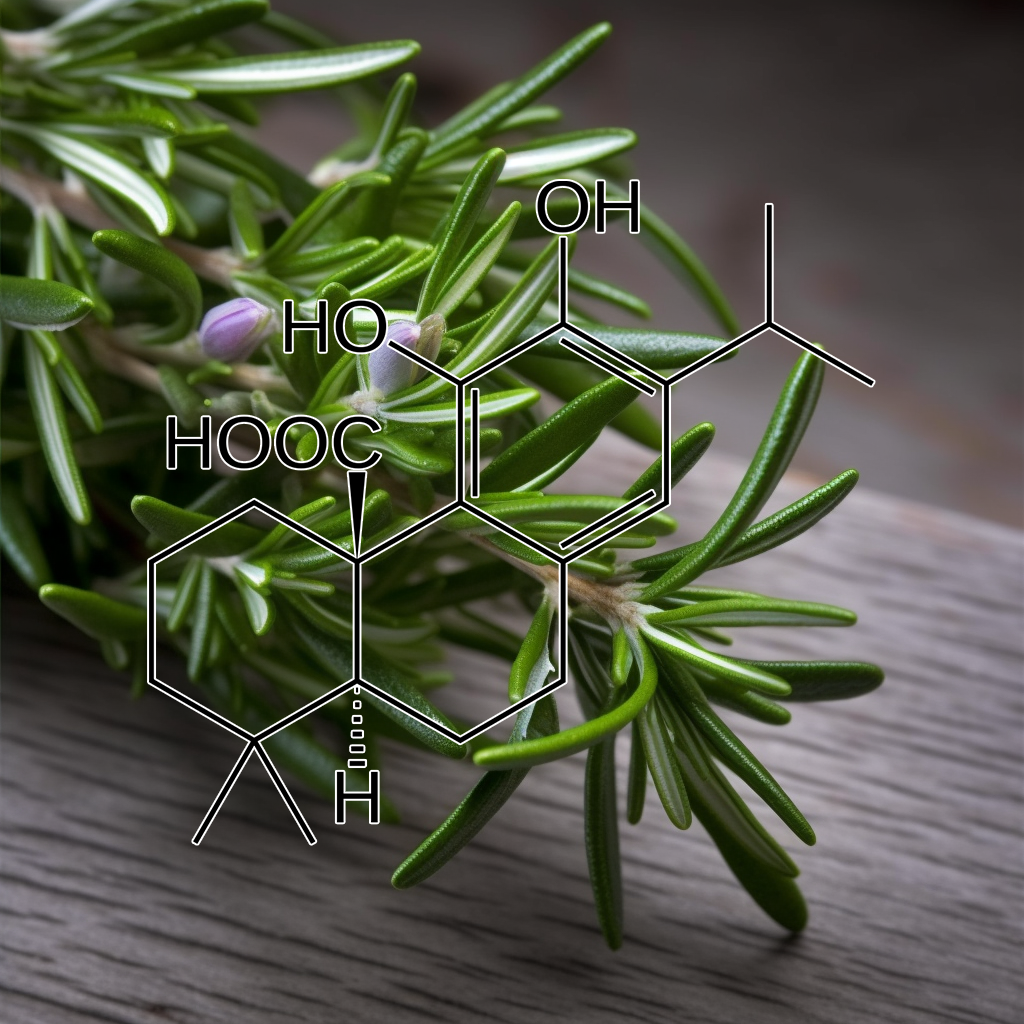


































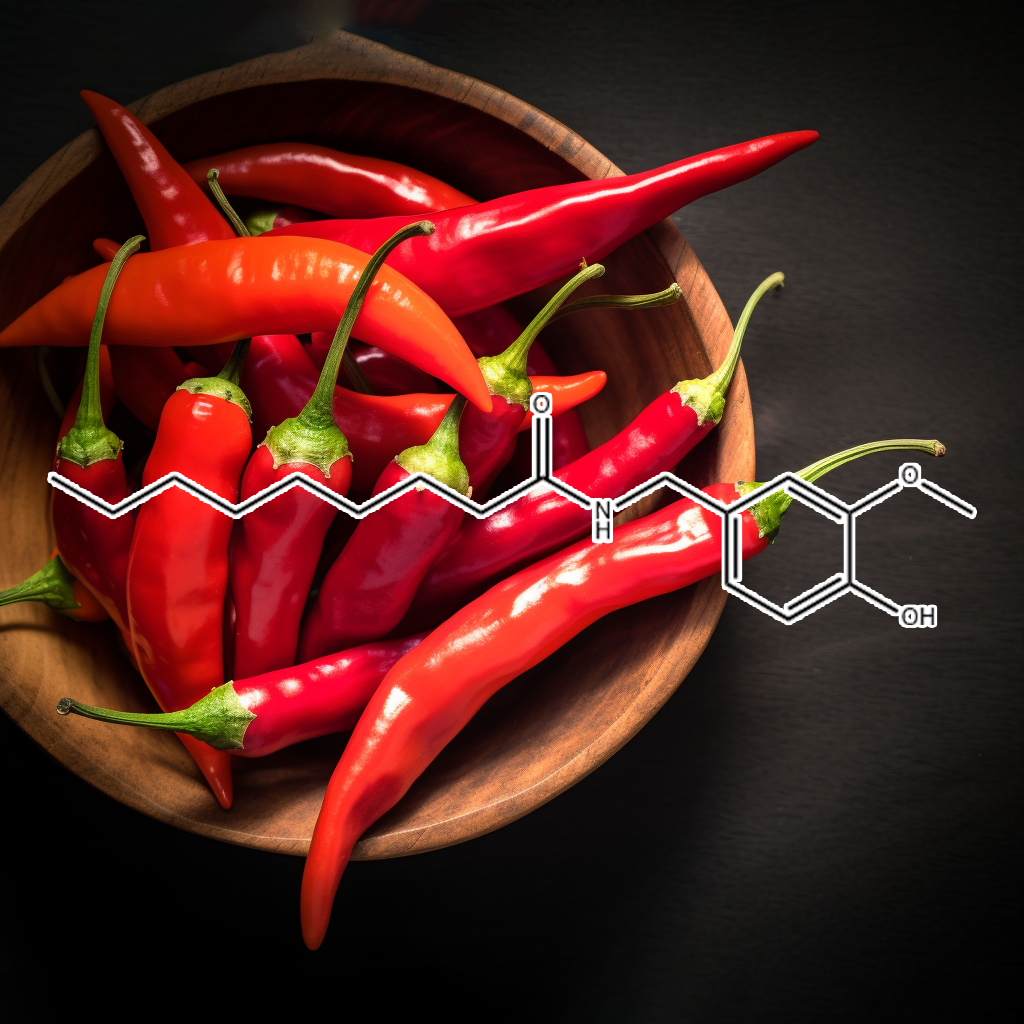

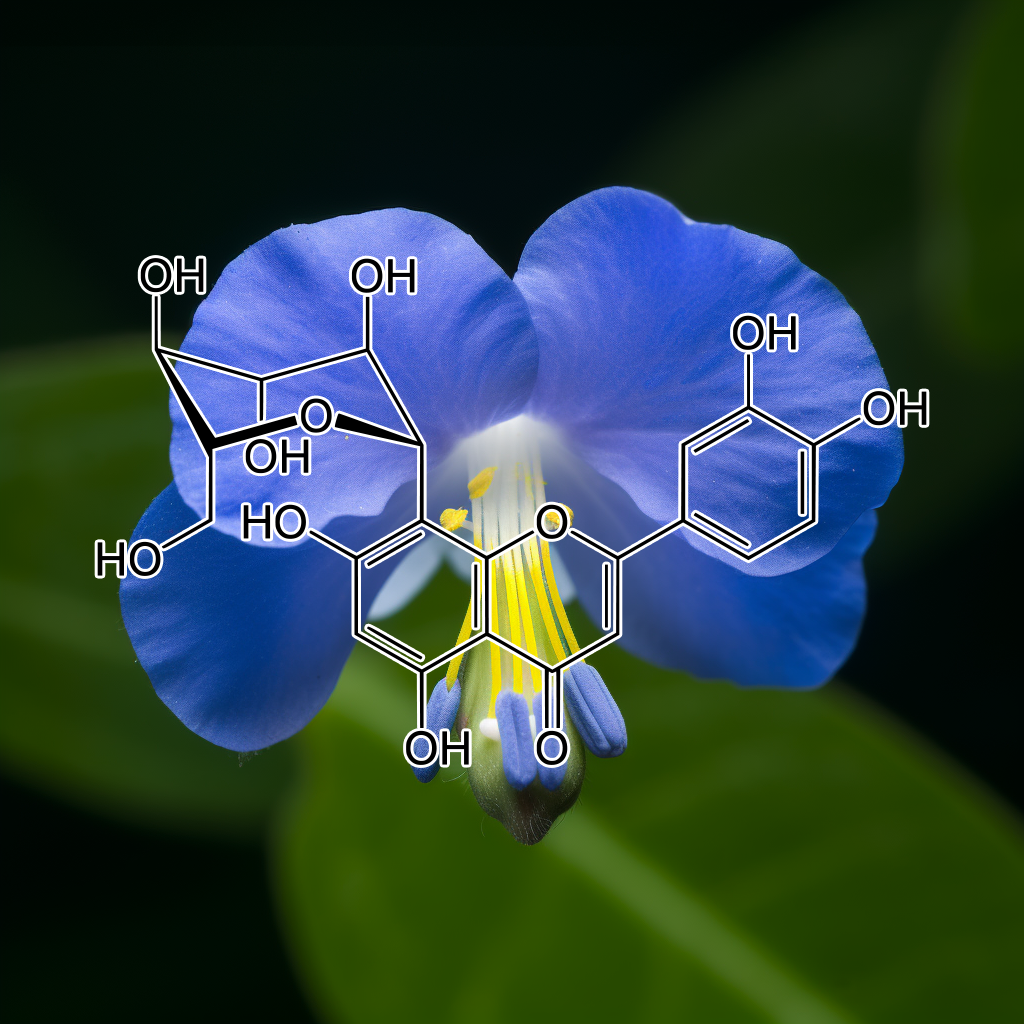




























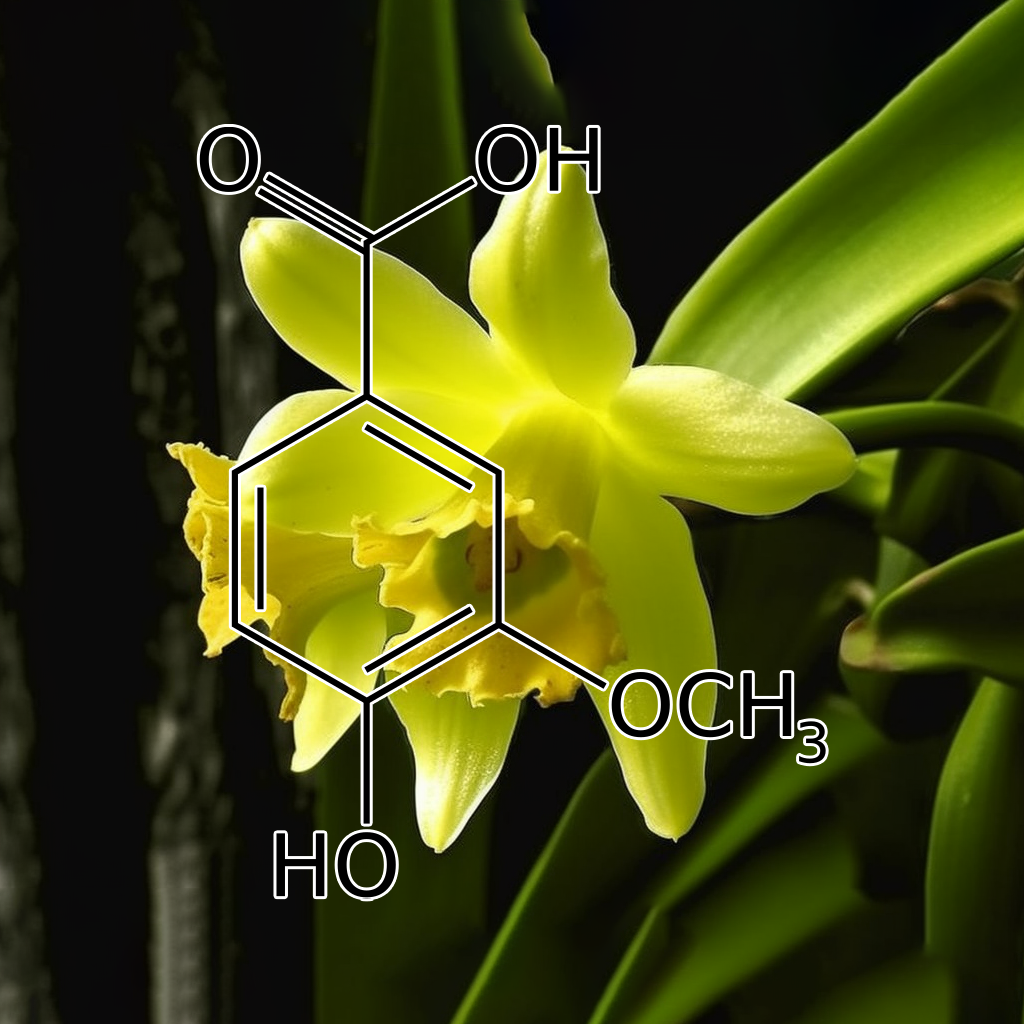




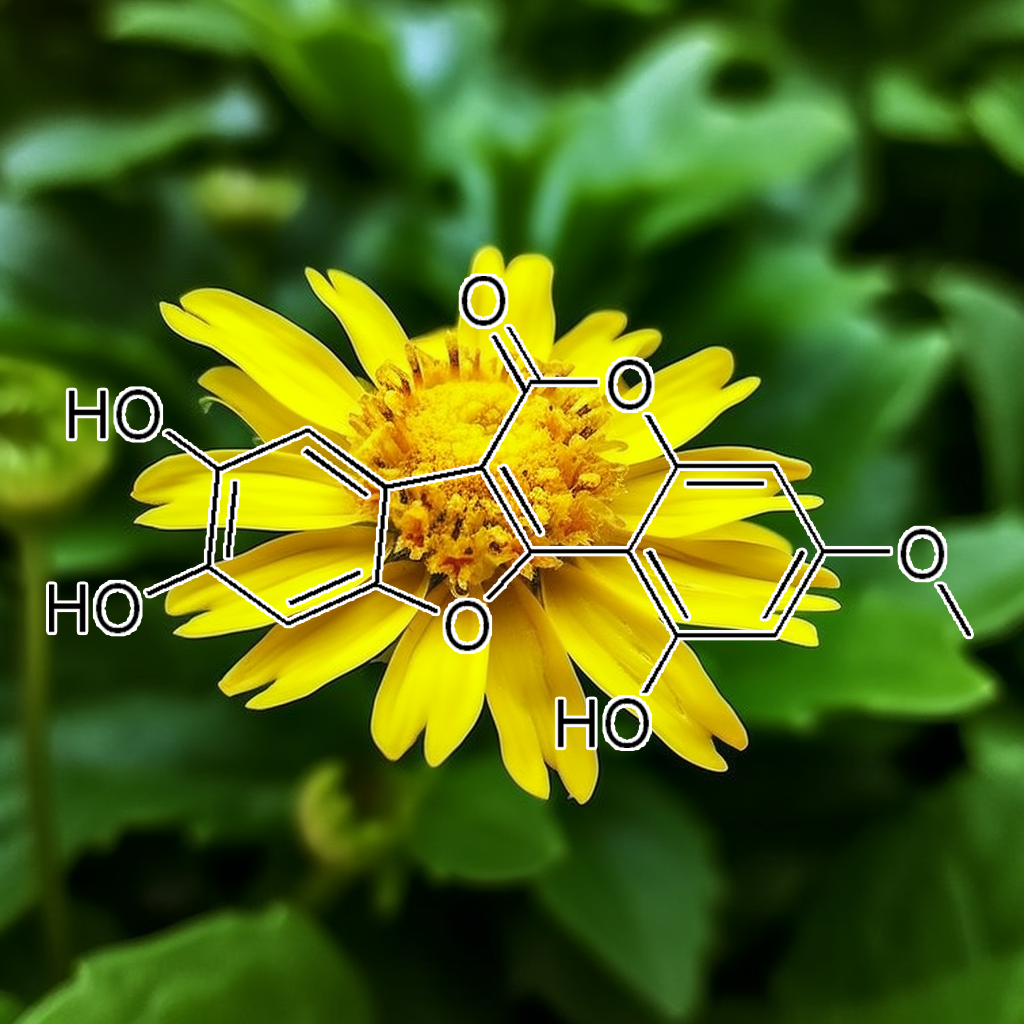




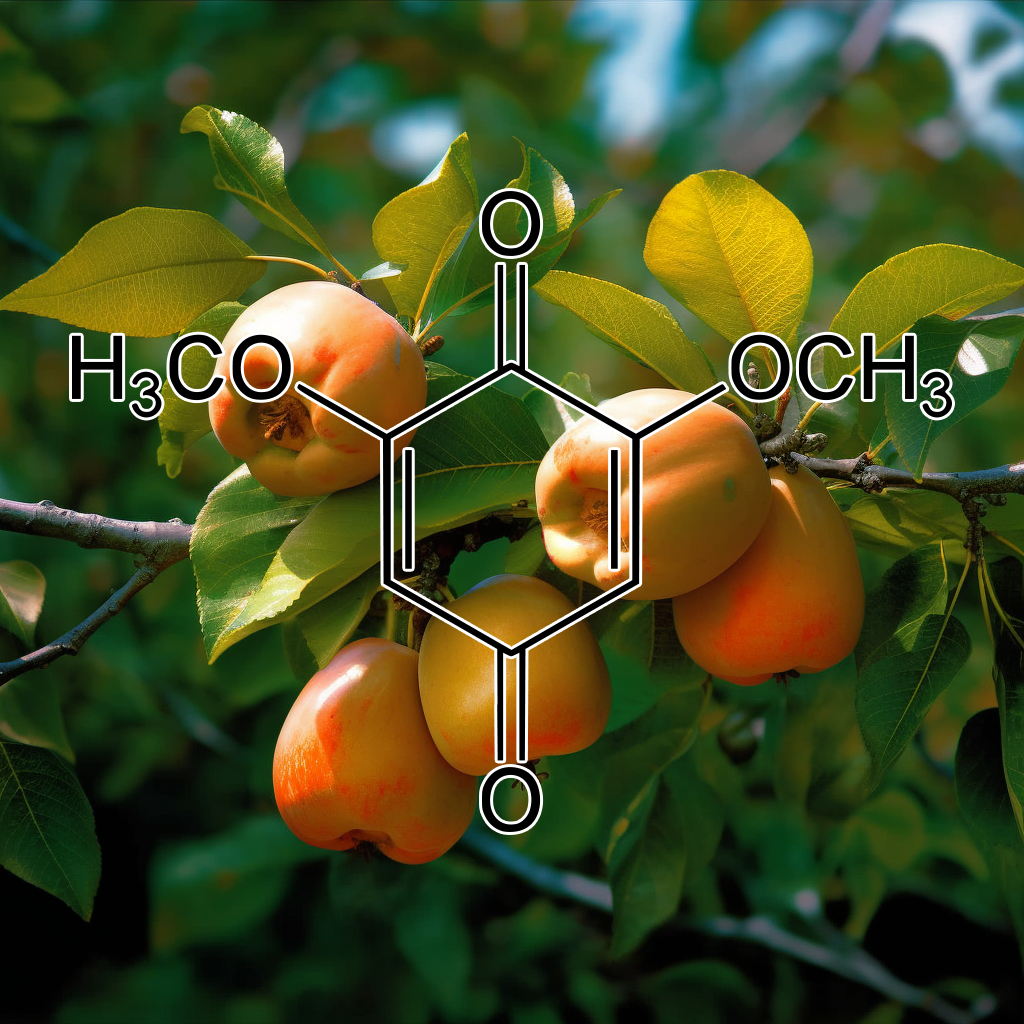
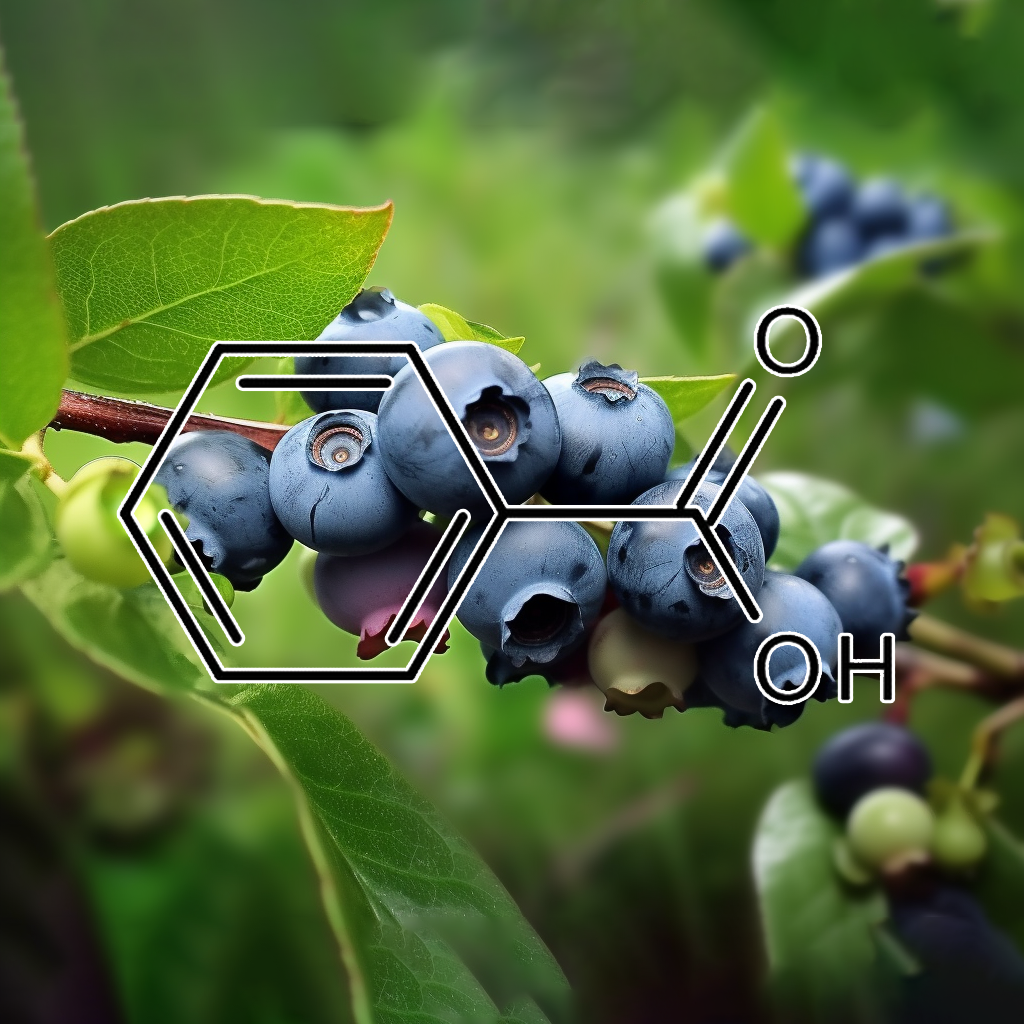








Rich Ryan –
If you have trouble losing weight, or if you diet and then gain it all back in a week, then this is the blend you need.
I’ve lost up to 1.5 lbs each day while fasting with this blend (and exercise). And if I keep taking it, then I don’t gain all the weight back after breaking fast. It’ll help you burn your love handles away and keep you from gaining them right back!
It has a great appetite suppressing effect as well.
Amazing Blend!
For ultimate appetite suppression and fat burning, pair with AutoPhagy and ThermoGenesis, and do 22/2 Intermittent Fasting.
To keep your mood up during fasting, pair with Trinity. And for more energy pair with Original Blend.
Boladale Abdulazeez –
I was introduced to this blend by my sister who has been on this blend for years, she encouraged me to give anti adipogenic and autography a try but i decided to buy the march combo for a start after my discussion with Gavin.
I orded the blend on the 7th of March, came in on the 10th and I started taking it immediately.
I started with weight 265 kg on the 10th of March and by the end of March I already lost about 45lbs, my skin glows and I feel lot stronger and healthier.
I still have alot to work on me, but am work in progress and am glad I am able to focus clearer, eat with control and feel stronger….I will add another review by the end of April.
Thank you Gavin, this blend truly works, and your customer services is amazing 100%…. excellent.
MS (verified owner) –
I have to say this is a remarkable product. I have been using this blend for over 3 months now, along with several other blends. I have been trying to adhere to the 22/2 protocol, but there are days which I can not. Even though, I have manage to reduce my body fat percentage, while increasing muscle in the gym. I’ve gotta put it down to this blend. It does what it says on the tin! I’ve noticed since I’ve run out about a month ago, eating the same diet, my body feels “flabbier”. My BF percentage even went up by 1%. The only thing I’ve changed is not having this blend.
Sometimes it takes not having something to realize it’s true benefit and hence why I wanted to write this review today.
I’ve got to hand it to Gavin, the blends are incredible. When you’ve been using them as long as I have, you become used to a bubble of light surrounding you, protecting you from bullshit.
Thanks!
Yvonne (verified owner) –
So I found the Interstellar site while researching Dry Fasting. I’ve been wet fasting for several years & seen tremendous benefits. I started intermittent fasting/OMAD after my health went to hell 5 years ago. I had been health conscious for 10 years, plant based, organic eating, no junk, juices, sweets, etc., after a Hyperinsulinanemia diagnosis, plus fungal acne, severe depression mainly brought on by stress I was able to turn it around in a couple months eating very low insulin/fasting/OMAD all that Gavin is a proponent of but I had never tried dry fasting as I honestly had no information on it.
Last year I was diagnosed with Generalized Anxiety Disorder, ADD and TBI (from childhood) from brain scans I did @ the Amen clinics. Which explained the struggles with depression & mental health despite going to great lengths to heal holistically and naturally. Having trained in holistic nutrition and being a natural researcher, I dug into the Interstellar site & I loved how well researched & science based everything was. It’s hard to find any solid information online about dry Fasting so I came away with so much info + about the Interstellar blends too. I’m a big proponent of medicinal plants and herbs so I knew their power but then seeing the hundreds of testimonials and then the weight loss group and everyone who was able to dump a huge amount of weight in no time I was like no way! I had to see it to believe it. I was out of the US but went to great lengths to order and get the blends.
I ordered the April sampler. My biggest goal was to lose weight but also brain health (focus/attention)… I had crippling anxiety. I was going through a terrible time emotionally and within a week most of the anxiety was gone! As in my stomach was tight day in and out and POOF! GONE! I was training hard at the time and the Pre-Workout blends (Nebula, Thermo, Anti Adipogenic & Matcha in black coffee had me going like superwoman 🦸🏾♀. My trainer, who had won several strongman championships couldn’t understand!
I got back stateside last month and I just had to try Rewire… I have struggled with my weight yo-yo-ing due to emotional eating/binging despite being so health focused (food addiction) so couldn’t wait to give it a go. Got a couple samplers and started Rewire this month along with the other cognitive enhancement blends (Trinity, Autonomous, Seven Sages, Luteolin) along with fasting as I work to heal my brain. I am loving the blends thus far and Gavin and the entire Interstellar’s team commitment to outstanding service, integrity and healing and the support of the community he has created. Truly inspiring!
Patty (verified owner) –
I’ve been taking the blends for over a year. LET ME TELL YOU, these are by far the BEST thing I stumbled upon. I have always been into alternative medicine and am never willing to take what big Pharma is pushing on me. I am a retired Registered Nurse and have pushed back against Pharma my entire career…weird right but my eyes were opened.
I have suffered with arthritis, pain, low energy but feeling of impending doom, thinning hair, and high blood pressure. Let me tell you….I tried many supplements to try and reverse these symptoms and nothing worked. Again by the Grace of God, I came across Gavin and his life saving blends. What a game changer!!
Here’s my results. By taking Peel and Spice for Arthritis, I have function back in my wrists and pain is under control. Low Energy and impending doom? GONE with NEBULA and TRINITY…I have never felt so calm and let things pass in my entire life! I use to be high strung and everything effected me. After having what I think was COVID..(never got tested) I started loosing my hair. How disturbing to me. I have been taking the hair blend and is thankfully growing back. Last but not least, EKG has brought my blood pressure into normal range. At every annual physical, my blood pressure was so high sometimes they didn’t want me to leave the clinic. This is a game changer for me. So happy I’m not in the critical zone anymore.
I could go on and on about the blends. Purge, Glucose Blocker, Anti Adipogenic, Thermo, and Autophagy for loosing weight…OFF THE CHARTS. I lost 10 pounds and have kept it off for over a year.
This is getting long so just try them….you’ll never go back! Thank You Gavin so much for your life saving and beautiful blends. They are filled with Love.
Patricia (verified owner) –
I’ve been taking the blends for over a year. LET ME TELL YOU, these are by far the BEST thing I stumbled upon. I have always been into alternative medicine and am never willing to take what big Pharma is pushing on me. I am a retired Registered Nurse and have pushed back against Pharma my entire career…weird right but my eyes were opened.
I have suffered with arthritis, pain, low energy but feeling of impending doom, thinning hair, and high blood pressure. Let me tell you….I tried many supplements to try and reverse these symptoms and nothing worked. Again by the Grace of God, I came across Gavin and his life saving blends. What a game changer!!
Here’s my results. By taking Peel and Spice for Arthritis, I have function back in my wrists and pain is under control. Low Energy and impending doom? GONE with NEBULA and TRINITY…I have never felt so calm and let things pass in my entire life! I use to be high strung and everything effected me. After having what I think was COVID..(never got tested) I started loosing my hair. How disturbing to me. I have been taking the hair blend and is thankfully growing back. Last but not least, EKG has brought my blood pressure into normal range. At every annual physical, my blood pressure was so high sometimes they didn’t want me to leave the clinic. This is a game changer for me. So happy I’m not in the critical zone anymore.
I could go on and on about the blends. Purge, Glucose Blocker, Anti Adipogenic, Thermo, and Autophagy for loosing weight…OFF THE CHARTS. I lost 10 pounds and have kept it off for over a year.
This is getting long so just try them….you’ll never go back! Thank You Gavin so much for your life saving and beautiful blends. They are filled with Love.
Pablo Arteaga –
2a. ACC ANTI ADIPOGENIC B3AR CERA. BLOCK. SENOLYTIC TRIG & HEAVY METAL DETOX, herby flavors with berry tints, especially for antiadip & trig. Dosed: ½ tsp (antiadip, senoly, & trig) ¼ tsp for Acc and B3AR. Personally, twice a day, mid day and afternoon.
2b. Desired Effect: to shed stubborn fat cells. Actual effect: loading up the system, too early to tell. Post effect: regular appetite, able to exercise and burn calories.
2c. Forethought: on their own, can be powerful catalysts for changes in weight. An increased hope in being able to kill generally difficult to reach fatty areas, ie thighs. Nothing will happen unless we improve our diets and consistently MOVE. (post note: lessened fatty areas! these are a handful of the blends to help you shred.)
Pablo Arteaga –
ACC ANTI ADIPOGENIC B3AR CERA. BLOCK. SENOLYTIC TRIG & HEAVY METAL DETOX, herby flavors with berry tints, especially for antiadip & trig. Dosed: ½ tsp (antiadip, senoly, & trig) ¼ tsp for Acc and B3AR. Personally, twice a day, mid day and afternoon. It should be noted that research is utterly vital before choosing your selection of blends to tackle the issue that pertains to your own situation!
Desired Effect: to shed stubborn fat cells, personally stored in thighs (genetics). Actual effect: a noticeable long term reduction in belly fat, tricep area fat and facial fat. Tangible results also include the replacement of “exczema cells” with healthy skin cells in the area above the eye, below the eyebrow (THANK SENOLYTIC). Post effect: regular appetite, able to exercise and burn calories. Tangible loss of fat cells in the target area (thighs). Over a period of 2 years, I’ve lost 20+ lbs worth of fat (155 to 130), and have been able to keep it off over the past 2 years thanks to the antiadipogenic blend offered by interstellar.
As for the Heavy Metal Detox, it is worth noting that a whole host of grocery products are contaminated with heavy metals, which are detrimental to vital organs such as your brain, heart and kidneys. To ensure maximum health and functionality to these organs, HMD is absolutely essential for complete restoration and functionality.
Forethought: on their own, these blends can be powerful catalysts for changes in weight. An increased hope in being able to kill generally difficult to reach fatty areas, ie thighs. Nothing will happen unless we improve our diets and consistently MOVE. HYPOTHESIS: Now, in order to stave off the accumulation of these fat cells (adipogenesis), regular exercise (stretching and cardio) must be maintained along with the ingestion of anti adipogenic and ceramide blocker. To recap; ACC, B3AR, & TRIG are extremely effective when in the process of shedding excessive FAT cells (adipose tissue, ceramides, lipids, triglycerides etc.). While Senescent (“dead”) cells can be destroyed with Senolytic. Then Ceramide blocker to prevent any accumulation of toxic fat and finally Antiadipogenic to prevent the accumulation of excessive basic adipose tissue. These 6 blends will help you to both lose and keep the fat off & HMD to remove the excess metals that may be weighing us down, which is found in commercial foods and certain water supplies. The detail is in the research, just know these are all beneficial blends to help you lose fat but not limited to these blends mentioned in this review.