
LUNG POWER
July 24, 2023
ANTI-FATIGUE
September 5, 2023GLP-1 ACTIVATOR
$275.00
Interstellar Blend ™ Ultimate GLP-1 Activator: Natural Metabolic Booster for Weight Loss & Blood Sugar Control Unlock the power of GLP-1 activation with our 200:1 concentrated supplement, designed to naturally modulate appetite, enhance insulin sensitivity, promote satiety via GLP-1 secretion, and support metabolic health. Featuring potent botanicals and bioactives like berberine and resveratrol, this formula mimics incretin effects to reduce hunger, stabilize blood glucose, aid fat metabolism, and improve cardiovascular wellness—ideal for diabetes management and sustainable weight loss without synthetic drugs.Key Benefits (Backed by Science):Appetite & Reward Regulation: Stimulates GLP-1 to curb cravings and overeating, as shown in studies on GLP-1 receptor activation in brain areas linked to hunger.
Anorexigenic Action: Enhances GLP-1 for delayed gastric emptying, reduced food intake, and improved glycemic control.
Addictive Behavior Support: Modulates GLP-1 pathways to potentially aid in managing substance-related disorders by influencing reward systems.
Weight Loss & Maintenance: Boosts GLP-1 for efficient fat burning, insulin release, and metabolic balance.
Cardiovascular Protection: GLP-1 agonists like those mimicked here reduce risks of heart disease, inflammation, and atherosclerosis.
Formulated from premium natural sources for jitter-free energy and long-term wellness. Consult a doctor before use.Full Ingredients List:1-Deoxynojirimycin
Acacia Tortilis Polysaccharide
Acarbose
Agave Tequilana Gto.
Allantoin
Amomi Fructus Rotundus
Anemarrhena Asphodeloides Bunge
Angelicae Sinensis Radix
Anthocyanin Delphinidin 3-Rutinoside
Arctium Lappa
Artemisia Dracunculus L.
Astragaloside A
Astragalus Polysacharin
Berberine
Berberis Vulgaris
Bupleurum Falcatum
Chebulae Fructus
Chlorogenic Acid
Cinnamomi Cortex
Cinnamtannin A2
Citrus Aurantium
Curcumin
Cynanchum Marnierianum
Delphinidin
Dendrobii Officmalis Caulis Polysaccharide
EGCG
Elaterin
Eucalyptus Citriodora
Fagopyrum Tataricum
Fructus Gardeniae
Ganoderma Lucidum
Ganoderma Lucidum Spore Powder
Gardenia Jasminoides
Genistein
Gentiana Scabra
Ginsenoside Rb1 Rg1 Rg5 T19 Rk3
Glycine Max
Grifolic Acid
Hawthorn Berry
Hispidulin
Hoodia Gordonii
Ilex Paraguariensis
Mangiferaindica
Momordica Charantia
Myricetin
Notoginsenoside Ft1
Peganum Harmala
Pinus Koraiensis
Prunus Africana
Pueraria Montana Var. Lobata (Willdenow) Maesen
Puerarin
Quercetin
Radix Ophiopogonis
Rehmanniae Radix
Resveratrol
Rhizoma Coptidis
Silymarin
Smallanthus Sonchifolius
Tithonia Diversifolia
Trichosanthes Kirilowii Maxim.
Triticum Aestivum
Zein Hydrolysate
Interstellar Blend ™ GLP-1 Activator




200:1 Concentration
Ingredients
- 1-Deoxynojirimycin
- Acacia Tortilis Polysaccharide
- Acarbose
- Agave Tequilana Gto.
- Allantoin
- Amomi Fructus Rotundus
- Anemarrhena Asphodeloides Bunge
- Angelicae Sinensis Radix
- Anthocyanin Delphinidin 3-Rutinoside
- Arctium Lappa
- Artemisia Dracunculus L.
- Astragaloside A
- Astragalus Polysacharin
- Berberine
- Berberis Vulgaris
- Bupleurum Falcatum
- Chebulae Fructus
- Chlorogenic Acid
- Cinnamomi Cortex
- Cinnamtannin A2
- Citrus Aurantium
- Curcumin
- Cynanchum Marnierianum
- Delphinidin
- Dendrobii Officmalis Caulis Polysaccharide
- EGCG
- Elaterin
- Eucalyptus Citriodora
- Fagopyrum Tataricum
- Fructus Gardeniae
- Ganoderma Lucidum
- Ganoderma Lucidum Spore Powder
- Gardenia Jasminoides
- Genistein
- Gentiana Scabra
- Ginsenoside Rb1 Rg1 Rg5 T19 Rk3
- Glycine Max
- Grifolic Acid
- Hawthorn Berry
- Hispidulin
- Hoodia Gordonii
- Ilex Paraguariensis
- Mangiferaindica
- Momordica Charantia
- Myricetin
- Notoginsenoside Ft1
- Peganum Harmala
- Pinus Koraiensis
- Prunus Africana
- Pueraria Montana Var. Lobata (Willdenow) Maesen
- Puerarin
- Quercetin
- Radix Ophiopogonis
- Rehmanniae Radix
- Resveratrol
- Rhizoma Coptidis
- Silymarin
- Smallanthus Sonchifolius
- Tithonia Diversifolia
- Trichosanthes Kirilowii Maxim.
- Triticum Aestivum
- Zein Hydrolysate
What does GLP-1 do?
GLP-1 Receptor Activation Modulates Appetite- and Reward-Related Brain Areas in Humans
In summary, we found that GLP-1 receptor agonists decreases hyperactivation in appetite- and reward-related brain regions, elicited by viewing food cues in obese T2DM and obese normoglycemic subjects, thus restoring an activation pattern that more closely resembles that of lean individuals. We found that these effects are GLP-1 receptor mediated and independent of circulating metabolic and hormonal factors. Our findings provide novel insights into the mechanisms by which GLP-1 regulates food intake and how GLP-1 receptor agonists cause weight loss.
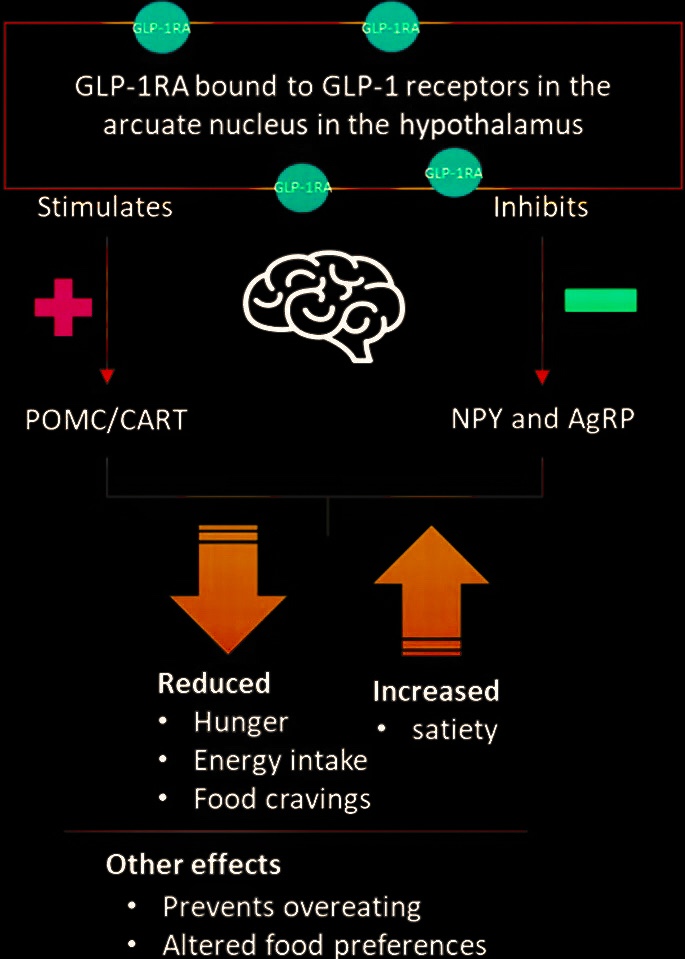
Anorexigenic Effects of GLP-1 and Its Analogues
GLP-1 receptors are expressed in the brain, especially in the regions responsible for the regulation of food intake, and intracerebroventricular injection of GLP-1 results in inhibition of food intake. Peripheral administration of GLP-1 dose-dependently enhances satiety and reduces food intake in normal and obese subjects as well as in type 2 diabetic patients.
The role of glucagon-like peptide 1 (GLP-1) in addictive disorders
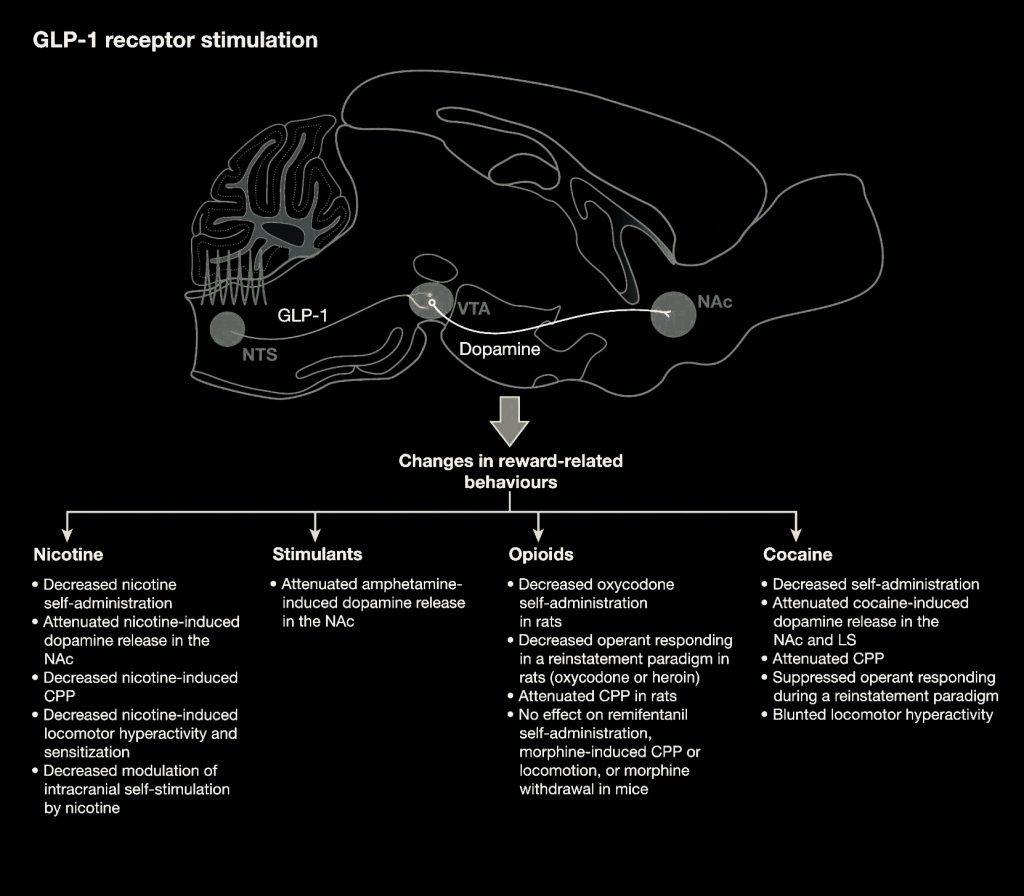
Drug, alcohol and tobacco use disorders are a global burden affecting millions of people. Despite decades of research, treatment options are sparse or missing, and relapse rates are high. Glucagon-like peptide 1 (GLP-1) is released in the small intestine, promotes blood glucose homeostasis, slows gastric emptying and reduces appetite. GLP-1 receptor agonists approved for treating Type 2 diabetes mellitus and obesity have received attention as a potential anti-addiction treatment. Studies in rodents and non-human primates have demonstrated a reduction in intake of alcohol and drugs of abuse.
The Efficacy of GLP-1 Analogues on Appetite Parameters, Gastric Emptying, Food Preference and Taste Among Adults with Obesity
GLP-1 analogues are effective obesity management therapy that decrease food intake and eventually reduce weight by suppressing appetite, reducing hunger, decreasing gastric emptying, and altering food preferences and taste.
Cardiovascular effects of Glucagon-like peptide 1 (GLP-1) receptor agonists
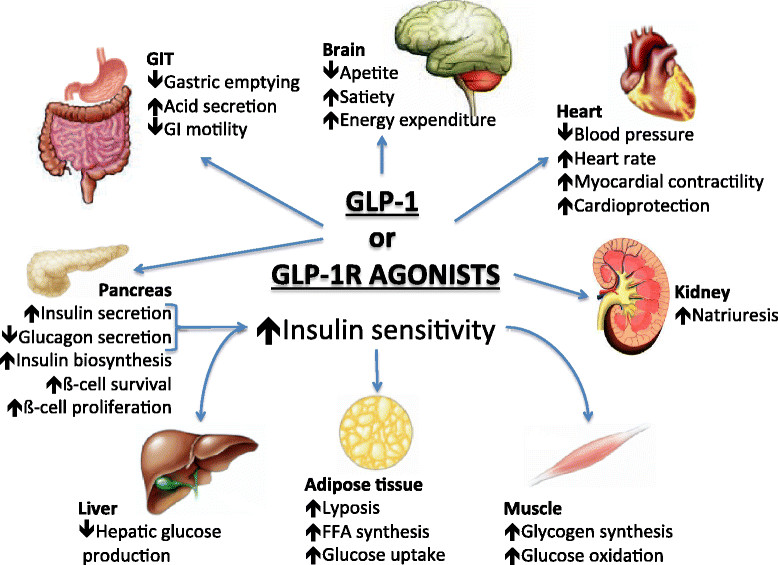
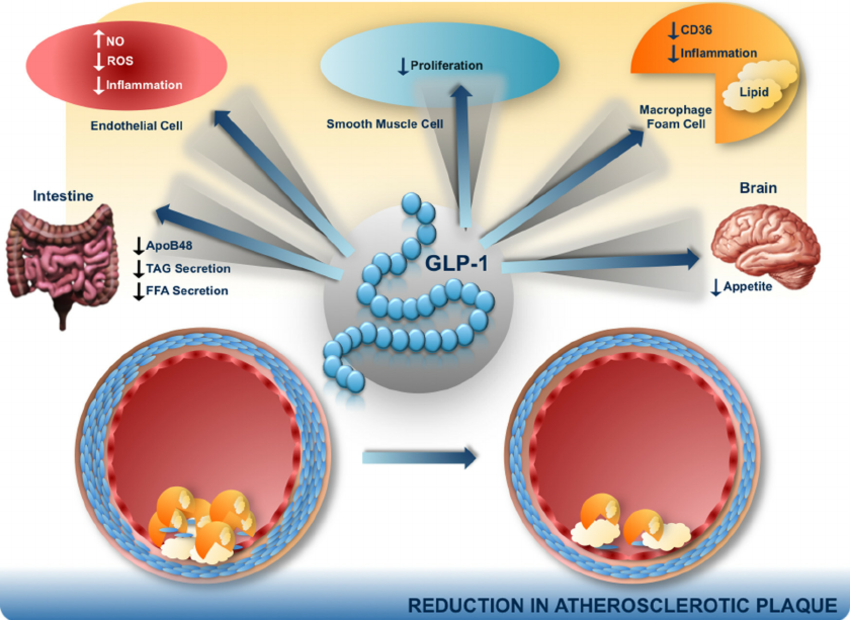
Patients with type 2 diabetes have a several-fold increased risk of developing cardiovascular disease when compared with nondiabetic controls. Myocardial infarction and stroke are responsible for 75% of all death in patients with diabetes, who present a 2-4× increased incidence of death from coronary artery disease. Patients with diabetes are considered for cardiovascular disease secondary prevention because their risk level is similar to that reported in patients without diabetes who have already suffered a myocardial infarction. More recently, with a better risk factors control, mainly in intensive LDL cholesterol targets with statins, a significant decrease in acute cardiovascular events was observed in population with diabetes. Together with other major risk factors, type 2 diabetes must be considered as an important cause of cardiovascular disease. Glucagon like peptide-1 receptor agonists represent a novel class of anti-hyperglycemic agents that have a cardiac-friendly profile, preserve neuronal cells and inhibit neuronal degeneration, an anti-inflammatory effect in liver protecting it against steatosis, increase insulin sensitivity, promote weight loss, and increase satiety or anorexia.
Ingredients & Science
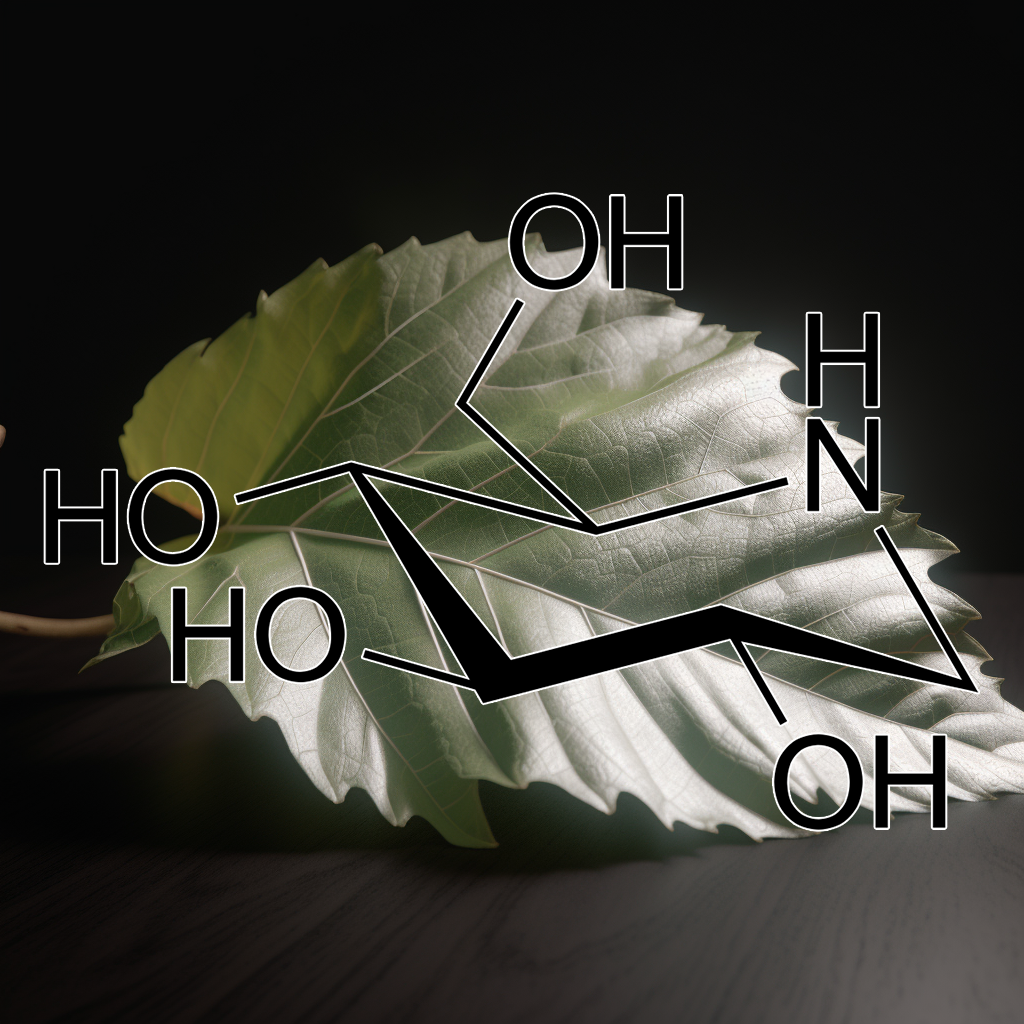
1-Deoxynojirimycin
The effects of miglitol on glucagon-like peptide-1 secretion and appetite sensations in obese type 2 diabetics
Background: Previous studies reported that administration of first generation alpha-glucosidase inhibitors (AGIs), such as voglibose or acarbose, produced exaggerated and sustained postprandial responses of glucagon-like peptide-1 (GLP-1), an incretin hormone from the enteroinsular axis, in healthy humans. Little is known about the postprandial release of GLP-1 after AGI therapy in diabetics. GLP-1 plays a role to mediate satiety. Any agent that substantially elevates GLP-1 levels may theoretically reduce hunger, increase satiation and limit food intake.
Objectives: This study was performed to analyse the effect of miglitol, a more potent second generation AGI with fewer gastrointestinal side-effects, on the regulation of meal-related GLP-1 secretion and on the change of insulin-glucose dynamics as well as the release of gastric inhibitory polypeptide (GIP), another incretin hormone, after stimulation by an ordinary meal in obese type-2-diabetic subjects. Miglitol’s subsequent influences on appetite sensations and food intake were also measured.
Design: In total, 8 obese type-2-diabetic women were randomized to receive treatment with 100 mg of miglitol or placebo three times a day for 2 days (six doses total) in a double-blind fashion. On day 3 of each treatment period (miglitol or placebo), measurements of GLP-1, GIP, insulin and glucose were taken periodically during 3 h after eating a 720 kcal breakfast. Appetite ratings with visual analogue scales (VASs) were used to assess ingestive behaviour hourly just before breakfast and hourly after for 6 h until immediately before lunch. The number of tuna sandwiches eaten at lunch was used to measure food consumption.
Results: The plasma GLP-1, glucose, insulin and GIP levels in response to the mixed meal were compared after the miglitol and placebo treatment. Miglitol effectively enhanced postprandial GLP-1 release and suppressed plasma GIP secretion. The ingestion of a mixed meal induced a remarkable rise in GLP-1 after miglitol as compared with placebo in overweight diabetic subjects. The meal-related rise in GLP-1 after miglitol was significantly greater at all time-points between 30 and 180 min than after the placebo. The postprandial incremental area under the curve for GLP-1 with miglitol treatment was about twofold that with the placebo. The GLP-1 level reached a maximum at 120 min after the mixed meal and steadily rose throughout the rest of the 3-h study period. In the miglitol-treated condition, the average caloric intake at lunch during a 30-min eating period was 12% lower (p < 0.05) as compared with that after the placebo in six out of the eight subjects who exhibited a GLP-1 rise after the breakfast meal by greater than 30% from the placebo-treated condition. Correspondingly, the average rating scores were significantly lower for hunger feelings and markedly greater for sensations of satiety under the miglitol treatment; beginning 2 and 3 h, respectively, before the lunch test.
Conclusions: Miglitol induced an enhanced and prolonged GLP-1 release at high physiological concentrations after ingesting an ordinary meal in glycaemic-controlled diabetics. The excessive postprandial GLP-1 elevation after miglitol therapy modified feeding behavior and food intake, and thereby has potential value in regulating appetite and stabilizing body weight in obese type-2-diabetic patients.
Mulberry leaf extract improves glycaemic response and insulaemic response to sucrose in healthy subjects
The mulberry leaf extract group exhibited a more balanced glucose response, with the participant’s blood glucose levels never dropping below the fasted level, whilst the placebo group showed a significantly lower blood glucose level at the 120-min time point (p < 0.001), levels lower than the fasted baseline. This transient reactive hypoglycaemia in the placebo group may be attributed to the rapid increase in blood glucose and the consequent spike in plasma insulin. In non-diabetic subjects, incretins are responsible for 50–70% of total insulin secretion.
Glucose-dependent insulinotropic peptide (GIP) and glucogon-like peptide-1 (GLP-1) both play an important role in glucose homeostasis[22], but the rate of release of these incretins differs depending on the stimulatory nutrient. GIP is synthesized by K-cells in the duodenum and jejenum and is very sensitive to glucose stimulation but has lower sensitivity to disaccharides and polysaccharides. GLP-1, which is produced by the L-cells that predominate at the distal end of the small intestine in the ileum, shows similar stimulatory sensitivity to glucose as to disaccharides and polysaccharides[23]. In healthy subjects, GIP is responsible for the majority of postprandial incretin-mediated insulin response[22] and in situations with lower amounts of free glucose, there would be a corresponding lower GIP-mediated insulin response.
This may explain the differences in blood glucose responses between the test groups in the current study and is in line with the established inhibitory effect that mulberry leaf extract has on glucosidase enzymes in the brush border[24]. In the placebo group, the sucrose test drink would be rapidly hydrolysed to glucose and fructose by glycosidase enzymes in the brush border of the intestine, resulting in a higher insulin release due to stimulation by both GIP and GLP-1, whilst in the Reducose® group, lower levels of free glucose would be available due to the inhibition of the hydrolysing enzymes, resulting in lower GIP levels. Early postprandial reactive hypoglycaemia, which occurs within 1–2 h of eating, can be caused by an exaggerated incretin effect as excessive amounts of GIP and GLP-1 are released through the sudden glucose loading, stimulating insulin exocytosis and upregulation of GLUT-4 channels, which results in a rapid lowering of blood glucose levels[25].

Acacia tortilis polysaccharide
Amelioration of Diabetes mellitus by modulation of GLP-1 via targeting alpha-glucosidase using Acacia tortilis polysaccharide in Streptozotocin-Nicotinamide induced diabetes in rats
Background: Polysaccharides decrease the glucose level by inhibiting α-glucosidase enzyme which further increases the level of GLP-1 (Glucagon-like peptide 1) to increase the insulin level as per earlier reports.
Objective: Similar hypothesis was designed in present study to investigate the α-glucosidase enzyme inhibition and involvement of GLP-1 in antidiabetic mechanism of Acacia tortilis polysaccharides (AEATP) in diabetic rats. Isolated polysaccharides were analyzed for their chemical nature by using HPLC and FTIR method.
Materials and Methods: Male albino wistar rats were divided into control, diabetic, diabetic + voglibose, diabetic + glimepiride, diabetic+250, 500, 1000 mg/kg of AEATP, diabetic + glimepiride + voglibose, diabetic + glimepiride+ 250, 500 and 1000 mg/kg AEATP, diabetic + GLP-1 antagonist+250, 500 and 1000 mg/kg AEATP. Plasma glucose, insulin and active GLP-1 levels were measured 15 min after OGTT. Fasting blood glucose, Plasma triglycerides, glycated hemoglobin (HbA1c), Fasting insulin, pancreatic insulin content, ileum and colon GLP-1 content were assessed at 5th week. Association of alpha-glucosidase was also assessed with GLP-1 and insulin.
Results: AEATP significantly attenuated hyperglycemia by increasing insulin level in plasma and pancreas and increased active GLP-1 as well as insulin level in diabetic rats after OGTT. GLP-1 content was significantly increased in ileum and colon by inhibiting alpha-glucosidase. Involvement of GLP-1 in antihyperglycemic effect of AEATP was confirmed by using GLP-1 antagonist. Moreover, AEATP significantly improved dyslipidemia in diabetic rats. HPLC analysis of A. tortilis polysaccharide comprised four specific monosaccharides (Rhamnose, Glucuronic acid, glucose and galactose) and FTIR spectrum shown band at 3430.6 cm-1 (O–H stretching), 2940.3 cm−1 (C–H linkage), 1630.4 cm−1 (carbonyl stretching), 1410 cm−1 (uronic acid) and 1030.5 cm−1 (glycosidic linkage).
Conclusion: It can be concluded that antidiabetic effect of AEATP is through the modulation of GLP-1 level in plasma and intestinal tissue via alpha glucosidase inhibition.
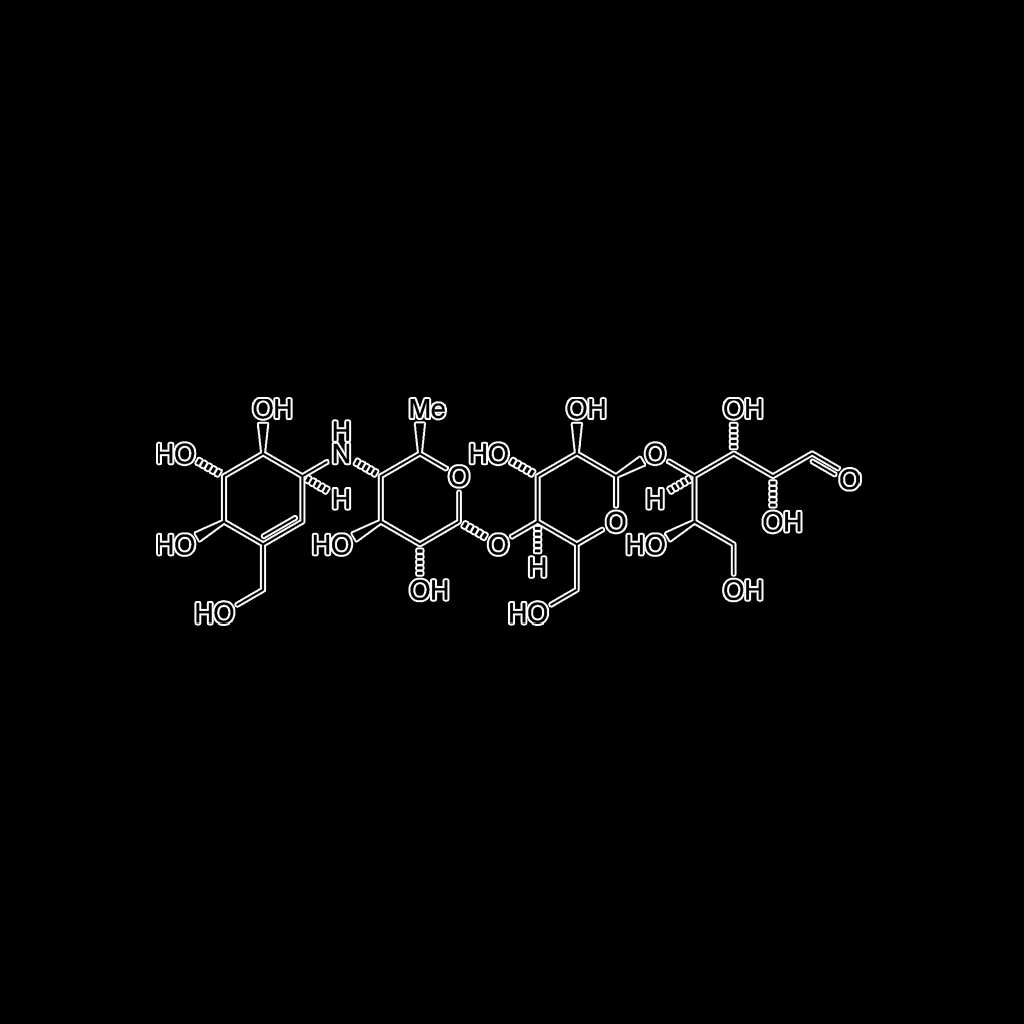
Acarbose
Effects of 24-week treatment with acarbose on glucagon-like peptide 1 in newly diagnosed type 2 diabetic patients: a preliminary report
Background: Treatment with the alpha-glucosidase inhibitor (AGI) acarbose is associated with a significant reduction the risk of cardiovascular events. However, the underlying mechanisms of this effect are unclear. AGIs were recently suggested to participate in stimulating glucagon-like peptide 1 (GLP-1) secretion. We therefore examined the effects of a 24-week treatment of acarbose on endogenous GLP-1, nitric oxide (NO) levels, nitric oxide synthase (NOS) activity, and carotid intima-media thickness (CIMT) in newly diagnosed patients with type 2 diabetes (T2D).
Methods: Blood was drawn from 24 subjects (14 male, 10 female, age: 50.7 ± 7.36 years, BMI: 26.64 ± 3.38 kg/m2, GHbA1c: 7.00 ± 0.74%) with drug-naïve T2D at 0 and 120 min following a standard mixed meal for the measurements of active GLP-1, NO and NOS. The CIMT was measured prior to and following 24 weeks of acarbose monotherapy (mean dose: 268 mg daily).
Results: Following 24 weeks of acarbose treatment, both fasting and postprandial plasma GLP-1 levels were increased. In patients with increased postprandial GLP-1 levels, serum NO levels and NOS activities were also significantly increased and were positively related to GLP-1 levels. Although the CIMT was not significantly altered following treatment with acarbose, a decreased CIMT was negatively correlated with increased GLP-1 levels.
Conclusion: Twenty-four weeks of acarbose monotherapy in newly diagnosed patients with T2D is associated with significantly increased levels of both fasting and postprandial GLP-1 as well as significantly increased NO levels and NOS activity for those patients in whom postprandial GLP-1 levels were increased. Therefore, the benefits of acarbose on cardiovascular risk may be related to its stimulation of GLP-1 secretion.

Agave tequilana
Physiological effects of dietary fructans extracted from Agave tequilana Gto. and Dasylirion spp.
Recent data reported that inulin-type fructans extracted from chicory roots regulate appetite and lipid/glucose metabolism, namely, by promoting glucagon-like peptide-1 (GLP-1) production in the colon. The Agave genus growing in different regions of Mexico also contains important amounts of original fructans, with interesting nutritional and technological properties, but only few data report their physiological effect when added in the diet.
Therefore, we decided to evaluate in parallel the effect of supplementation with 10 % agave or chicory fructans on glucose and lipid metabolism in mice. Male C57Bl/6J mice were fed a standard (STD) diet or diet supplemented with Raftilose P95 (RAF), fructans from Agave tequilana Gto. (TEQ) or fructans from Dasylirion spp. (DAS) for 5 weeks. The body weight gain and food intake in mice fed fructans-containing diets were significantly lower than the ones of mice fed the STD diet, TEQ leading to the lowest value. Serum glucose and cholesterol were similarly lower in all fructans-fed groups than in the STD group and correlated to body weight gain.
Only RAF led to a significant decrease in serum TAG. As previously shown for RAF, the supplementation with agave fructans (TEQ and DAS) induced a higher concentration of GLP-1 and its precursor, proglucagon mRNA, in the different colonic segments, thus suggesting that fermentable fructans from different botanical origin and chemical structure are able to promote the production of satietogenic/incretin peptides in the lower part of the gut, with promising effects on glucose metabolism, body weight and fat mass development.
Agavins from Agave angustifolia and Agave potatorum affect food intake, body weight gain and satiety-related hormones (GLP-1 and ghrelin) in mice
We evaluated the energy intake daily and weight gain every week. At the end of the experiment, portal vein blood samples as well as intestinal segments and the stomach were collected to measure glucagon-like peptide-1 (GLP-1) and ghrelin using RIA and ELISA kits, respectively. Colon SCFAs were measured using gas chromatography. The energy intake, body weight gain, and triglycerides were lower in the fructan-fed mice than in the STD-fed mice. The AASDP, APSDP, and RSE diets increased the serum levels of GLP-1 (40, 93, and 16%, respectively vs. STD) (P ≤ 0.05), whereas ghrelin was decreased (16, 38, and 42%, respectively) (P ≤ 0.05). Butyric acid increased significantly in the APSDP-fed mice (26.59 mmol g(-1), P ≤ 0.001) compared with that in the AASDP- and RSE-fed mice. We concluded that AASDP and APSDP are able to promote the secretion of the peptides involved in appetite regulation, which might help to control obesity and its associated metabolic disorder.

Allantoin
Antidiabetic Effects of Yam (Dioscorea batatas) and Its Active Constituent, Allantoin, in a Rat Model of Streptozotocin-Induced Diabetes
The objective of this study was to investigate the therapeutic efficacies of crude yam (Dioscorea batatas) powder (PY), water extract of yam (EY), and allantoin (the active constituent of yam) in streptozotocin (STZ)-induced diabetic rats with respect to glucose, insulin, glucagon-like peptide-1 (GLP-1), C-peptide, glycated hemoglobin (HbAlc), lipid metabolism, and oxidative stress. For this purpose, 50 rats were divided into five groups: normal control (NC), diabetic control (STZ), and STZ plus treatment groups (STZ + PY, STZ + EY, and STZ + allantoin). After treatment for one-month, there was a decrease in blood glucose: 385 ± 7 in STZ, 231 ± 3 in STZ + PY, 214 ± 11 in STZ + EY, and 243 ± 6 mg/dL in STZ + allantoin, respectively.
The treatment effectively ameliorated antioxidant stress as shown by a significant decrease (p < 0.001) in malondialdehyde (100% as 7.25 ± 0.11 nmol/mL, 87%, 86%, and 85%) together with increases (p < 0.01) in superoxide dismutase (100% as 167 ± 6 IU/mL, 147%, 159%, and 145%) and reduced glutathione (100% as 167 ± 6 nmol/mL, 123%, 141%, and 140%). The results indicate that yam and allantoin have antidiabetic effects by modulating antioxidant activities, lipid profiles and by promoting the release of GLP-1, thereby improving the function of β-cells maintaining normal insulin and glucose levels.
Allantoin attenuates deficits of behavioural and motor nerve conduction in an animal model of cisplatin-induced neurotoxicity in rats
Reports suggest that allantoin exerts antidiabetic activity in streptozotocin-induced diabetic rats by decreasing the levels of fasting blood glucose and HbAlc, while conversely increasing the levels of insulin, GLP-1, and C-peptide. In recent reports allantoin treatment increased the serum antioxidant activities of tGSH, GSH, and SOD, but decreased the levels of MDA and GSSG.5 These findings suggested that allantoin treatment may be able to ameliorate the effects of chronic oxidative stress in β-cells and other bodily organs.
Allantoin has also been shown to exert anti-inflammatory effects. IL-6, a multifunctional pro-inflammatory cytokine, affects the secretion of GLP-1 by intestinal L cells. The levels of circulating GLP-1 have been found to correlate with the concentration of systemic IL-6 and also with the concentrations of other markers of inflammation, suggesting that the regulation of GLP-1 is inflammation dependent. It is thus worth noting that treatment with allantoin may promote the release of GLP-1 and improve the function of β-cells, thereby maintaining insulin and glucose levels.21

Amomi Fructus
Norlignans as potent GLP-1 secretagogues from the fruits of Amomum villosum
The dried fruit of Amomum villosum (Amomi Fructus) is an important spices and traditional Chinese medicine. In this study, the EtOH extract of Amomi Fructus was revealed with hypoglycemic effects on db/db mice by increasing plasma insulin levels. After extracted with EtOAc, the EtOAc fraction showed increased activity in stimulating glucagon-like peptide-1 (GLP-1) secretion compared with the EtOH extract.
In order to clarify the antidiabetic constituents, four undescribed norlignans, amovillosumins A‒D, were isolated from the EtOAc fraction, and the subsequent chiral resolution yielded three pairs of enantiomers. Their structures were determined by extensive spectroscopic data (1D and 2D NMR, HRESIMS, IR, UV and [α]D) and ECD calculations. Amovillosumins A and B significantly stimulated GLP-1 secretion by 375.1% and 222.7% at 25.0 μM, and 166.9% and 62.7% at 12.5 μM, representing a new type of GLP-1 secretagogues.

Anemarrhena asphodeloides
Anemarrhena Asphodeloides GLP-1 : A Natural Solution for Diabetes Management
Anemarrhena Asphodeloides GLP-1 is a natural solution that shows promise in diabetes management. It offers benefits such as improved blood sugar control, weight management, and potential cardiovascular benefits. However, it is important to remember that Anemarrhena Asphodeloides GLP-1 is not a replacement for insulin and should be used under the guidance of a healthcare professional. If you are interested in exploring Anemarrhena Asphodeloides GLP-1 as a treatment option, consult with your doctor to determine if it is right for you.

Angelicae Sinensis
Angelica dahurica Extracts Improve Glucose Tolerance through the Activation of GPR119
G protein-coupled receptor (GPR) 119 is expressed in pancreatic β-cells and intestinal L cells, and is involved in glucose-stimulated insulin secretion and glucagon-like peptide-1 (GLP-1) release, respectively. Therefore, the development of GPR119 agonists is a potential treatment for type 2 diabetes. We screened 1500 natural plant extracts for GPR119 agonistic actions and investigated the most promising extract, that from Angelica dahurica (AD), for hypoglycemic actions in vitro and in vivo. Human GPR119 activation was measured in GeneBLAzer T-Rex GPR119-CRE-bla CHO-K1 cells; intracellular cAMP levels and insulin secretion were measured in INS-1 cells; and GLP-1 release was measured in GLUTag cells. Glucose tolerance tests and serum plasma insulin levels were measured in normal C57BL6 mice and diabetic db/db mice. AD extract-treated cells showed significant increases in GPR119 activation, intracellular cAMP levels, GLP-1 levels and glucose-stimulated insulin secretion as compared with controls.
In normal mice, a single treatment with AD extract improved glucose tolerance and increased insulin secretion. Treatment with multiple doses of AD extract or n-hexane fraction improved glucose tolerance in diabetic db/db mice. Imperatorin, phellopterin and isoimperatorin were identified in the active fraction of AD extract. Among these, phellopterin activated GPR119 and increased active GLP-1 and insulin secretion in vitro and enhanced glucose tolerance in normal and db/db mice. We suggest that phellopterin might have a therapeutic potential for the treatment of type 2 diabetes.

Anthocyanin Delphinidin 3-Rutinoside
The Anthocyanin Delphinidin 3-Rutinoside Stimulates Glucagon-Like Peptide-1 Secretion in Murine GLUTag Cell Line via the Ca2+/Calmodulin-Dependent Kinase II Pathway – PMC
Glucagon-like peptide-1 (GLP-1) is an incretin hormone secreted from enteroendocrine L-cells. Although several nutrients induce GLP-1 secretion, there is little evidence to suggest that non-nutritive compounds directly increase GLP-1 secretion. Here, we hypothesized that anthocyanins induce GLP-1 secretion and thereby significantly contribute to the prevention and treatment of diabetes. Delphinidin 3-rutinoside (D3R) was shown to increase GLP-1 secretion in GLUTag L cells. The results suggested that three hydroxyl or two methoxyl moieties on the aromatic ring are essential for the stimulation of GLP-1 secretion. Notably, the rutinose moiety was shown to be a potent enhancer of GLP-1 secretion, but only in conjunction with three hydroxyl moieties on the aromatic ring (D3R). Receptor antagonist studies revealed that D3R-stimulates GLP-1 secretion involving inositol 1,4,5-trisphosphate receptor-mediated intracellular Ca2+mobilization.
Treatment of GLUTag cells with a Ca2+/calmodulin-dependent kinaseII (CaMKII) inhibitor (KN-93) abolished D3R-stimulated GLP-1 secretion. In addition, treatment of GLUTag cells with D3R resulted in activation of CaMKII. Pre-treatment of cells with a G protein-coupled receptor (GPR) 40/120 antagonist (GW1100) also significantly decreased D3R-stimulated GLP-1 secretion. These observations suggest that D3R stimulates GLP-1 secretion in GLUTag cells, and that stimulation of GLP-1 secretion by D3R is mediated via Ca2+-CaMKII pathway, which may possibly be mediated by GPR40/120.
These findings provide a possible molecular mechanism of GLP-1 secretion in intestinal L-cells mediated by foods or drugs and demonstrate a novel biological function of anthocyanins in regards to GLP-1 secretion.

Arctium lappa
Studies available in scientific literature provide evidence of the A. lappa beneficial effects on health. In particular, the plant appears useful in the management of T2DM; thus, folk uses are strongly supported by scientific evidence. In particular, A. lappa has been shown to improve glucose homeostasis acting with different mechanisms, including (a) increased glucose uptake in muscle, (b) reduced glucose output induced by glucagon in hepatocytes, (c) activation of AMPK pathways, (d) inhibition of α‐glucosidase activity, and (d) stimulation of GLP‐1 release.
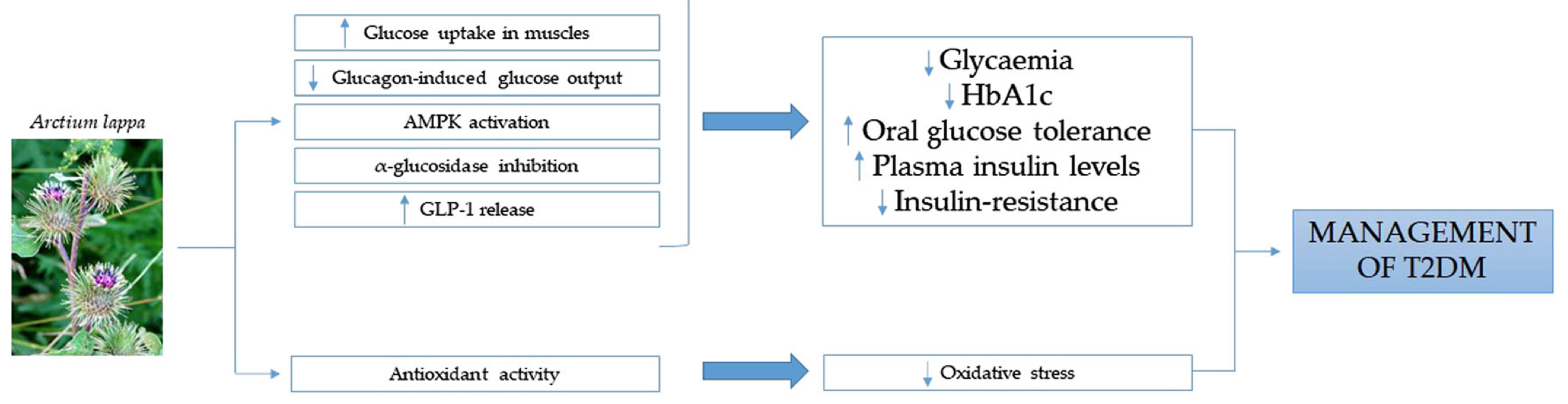
An increase in GLP‐1 release has been reported to be an efficacious therapeutic strategy for the management of T2DM (Ezcurra, Reimann, Gribble & Emery, 2013; Troke, Tan & Bloom, 2014). This action might be also considered for medical plants and certain foods. In particular, a recent narrative review highlights the effects of natural bitter compounds in stimulating intestinal bitter taste receptors (taste receptor type 2, TAS2R), resulting in increased incretin release, including GLP-1 (Barrea et al., 2019). Potentially, thus, the increased GLP-1 release observed by Xu and colleagues could be explained by an interaction of these compounds with TAS2R; however, further studies are needed.

Artemisia dracunculus L.
An Extract of Artemisia dracunculus L. stimulates insulin secretion from β cells, activates AMPK and suppresses inflammation
β cells have been known to have a capacity for replication in rodents and in humans as well. A variety of peptides such as INGAP, a peptide fragment of the pancreatic REG protein, GLP-1 and the GLP-1 receptor agonist exendin-4, the combination of betacellulin and activin A, and the combination of EGF and gastrin has been shown to stimulate replication/neogenesis of β cells in rodents. Also, GLP-1/exendin-4 has incretin effects, enhances insulin secretion and has anti-apoptotic effects (Bonner-Weir and Weir, 2005).
Artemisia dracunculus L. extract ameliorates insulin sensitivity by attenuating inflammatory signalling in human skeletal muscle culture
The studies reported here were undertaken to examine the antihyperglycemic activity of an ethanolic extract of Artemisia dracunculus L., called Tarralin in diabetic and non-diabetic animals. In genetically diabetic KK-A(gamma) mice, Tarralin treatment by gavage (500 mg/kg body wt./day for 7 days) lowered elevated blood glucose levels by 24% from 479+/-25 to 352+/-16 mg/dl relative to control animals. In comparison, treatment with the known antidiabetic drugs, troglitazone (30 mg/kg body wt./day) and metformin (300 mg/kg body wt./day), decreased blood glucose concentrations by 28% and 41%, respectively.
As a possible mechanism, Tarralin was shown to significantly decrease phosphoenolpyruvate carboxykinase (PEPCK) mRNA expression by 28% in STZ-induced diabetic rats. In non-diabetic animals, treatment with Tarralin did not significantly alter PEPCK expression, blood glucose or insulin concentrations. The extract was also shown to increase the binding of glucagon-like peptide (GLP-1) to its receptor in vitro. These results indicate that Tarralin has antihyperglycemic activity and a potential role in the management of diabetic states.
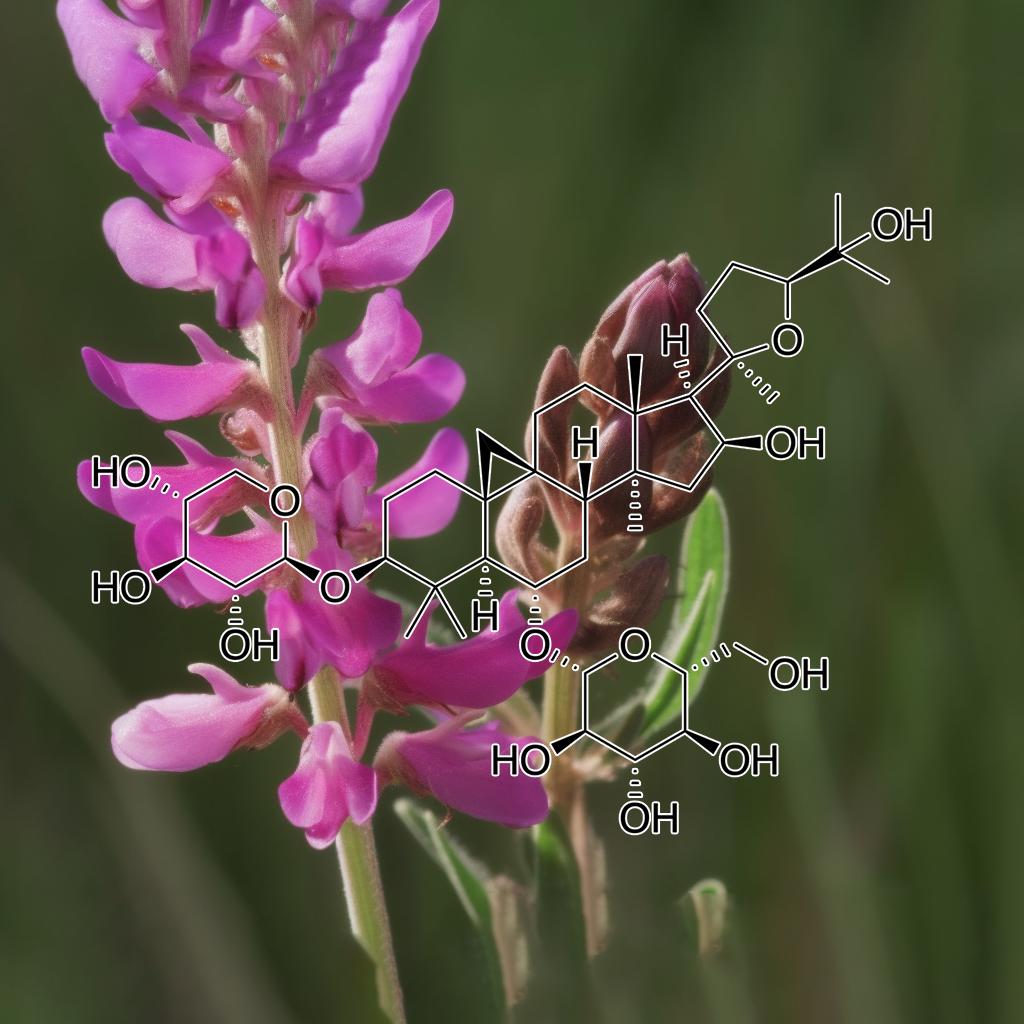
Astragalus
Astragalus polysaccharide alleviates type 2 diabetic rats by reversing the glucose transporters and sweet taste receptors/GLP-1 /GLP-1 receptor signaling pathways in the intestine-pancreatic axis
The present study revealed that Astragalus polysaccharide (APS) alleviated the symptoms of type 2 diabetes mellitus (T2DM) rats, improved the histopathology of pancreas and promoted insulin secretion. Gene expression analysis found that APS significantly increased the expressions of Glucagon-like peptide 1 and sweet taste receptors (STRs) signaling molecules, and decreased the expressions of glucose transporters of SGLT-1 and GLUT2 in the intestine of T2DM rats. Furthermore, APS significantly increased the expressions of GLP-1 and STRs signaling molecules in the pancreas of T2DM rats, as well as the expression of GLP-1 receptor signaling molecules.
Conversely, APS remarkably decreased the expressions of GLUT2 and KCNJ11 in the pancreas of T2DM rats. These results suggested that the physiological roles of STRs in the intestine and pancreas were involved in glucose uptake and insulin secretion, respectively. APS alleviated T2DM rats by reversing expressions of glucose transporters and STRs/GLP-1 /GLP-1 R pathways in the intestine-pancreatic axis of T2DM rats.

Astragalus Polysaccharide
Effect of Astragalus Polysaccharide on Sweet Taste Receptor Pathway in Intestine of Rat Model Induced by High-sugar and High-fat Diet
Method: SD rats were randomly divided into normal group, high-sugar and high-fat group and astragalus polysaccharide group. Rats in high-sugar and high-fat group and astragalus polysaccharide groups were fed with high-sugar and high-fat diet for 16 weeks, while rats in astragalus polysaccharide group were fed with APS (0.7 g·kg-1, per day) for 8 weeks during this period. Serum samples were collected to determine the levels of fasting blood glucose, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C). Intestinum tenue was collected to determine mRNA expressions of T1R2/T1R3, α-gustducin (Gα gust), transient receptor potential cation channel subfamily member 5 (TRPM5) and proglucagon (PG) gene by Real-time PCR, and protein expressions of T1R2, Gα gust and glucagon-like peptide-1 (GLP-1) protein by Western blot.
Result: Rats in high-sugar and high-fat group had significantly higher levels of TC, TG and LDL-C, and lower HDL-C level in serum than those in normal group (Pα gust and PG genes in intestine, were significantly down-regulated in high-sugar and high-fat group (PPα gust, TRPM5 and PG genes in intestine were significantly up-regulated in astragalus polysaccharide group (Pα gust and GLP-1 protein expressions was consistent with that of T1R2, Gα gust and GLP-1 mRNA expressions. Protein expressions of T1R2, Gα gust and GLP-1 and mRNA expression of T1R3 were significantly lower in astragalus polysaccharide group than those of control group (P
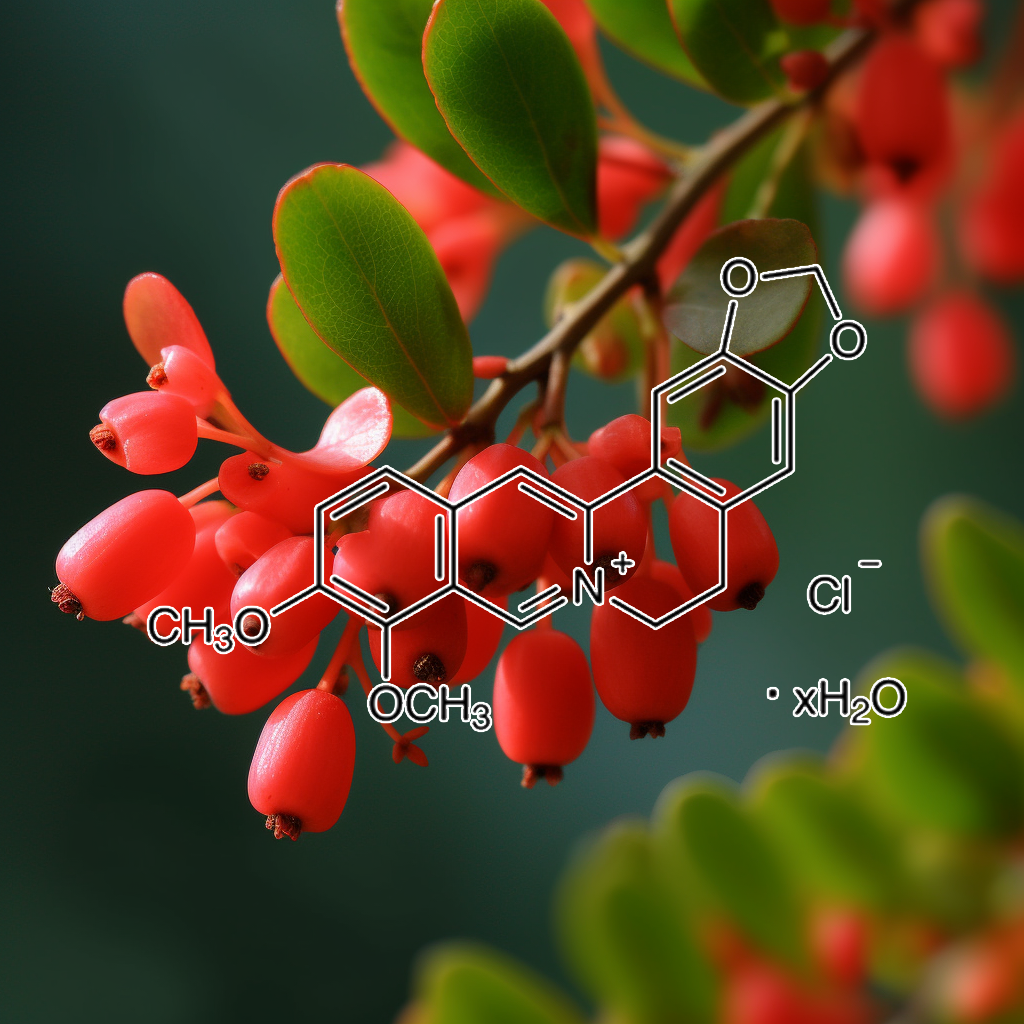
Berberine
Boosting GLP-1 by Natural Products
The prevalence of diabetes mellitus is growing rapidly. Diabetes is the underlying cause of many metabolic and tissue dysfunctions, and, therefore, many therapeutic agents have been developed to regulate the glycemic profile. Glucagon-like peptide-1 (GLP-1) receptor agonists are a newly developed class of antidiabetic drugs that have potent hypoglycemic effects via several molecular pathways. In addition to synthetic GLP-1 receptor agonists, some evidence suggests that natural products may have modulatory effects on GLP-1 expression and secretion. In the current study, we conclude that certain herbal-based constituents, such as berberine, tea, curcumin, cinnamon, wheat, soybean, resveratrol, and gardenia, can exert an influence on GLP-1 release.
Berberine moderates glucose metabolism through the GnRH-GLP-1 and MAPK pathways in the intestine
Background: Berberine is known to improve glucose and lipid metabolism disorders, but it poorly absorbed into the blood stream from the gut. Therefore, the exact underlying mechanism for berberine is still unknown. In this study, we investigated the effect of berberine on glucose metabolism in diabetic rats and tested the hypothesis that berberine acts directly in the terminal ileums.
Methods: Rats were divided into a control group, diabetic group (DM), low dose of berberine group (BerL) and high dose of berberine group (BerH). Ileum samples were analyzed using a Roche NimbleGen mRNA array, qPCR and immunohistochemistry.
Results: We found that 8 weeks of treatment with berberine significantly decreased fasting blood glucose levels. An oral glucose tolerance test (OGTT) showed that blood glucose was significantly reduced in the BerL and BerH groups before and at 30 min, 60 min and 120 min after oral glucose administration. Plasma postprandial glucagon-like peptide-1 (GLP-1) levels were increased in the berberine-treated groups. The ileum from the BerH group had 2112 genes with significantly changed expression (780 increased, 1332 decreased). KEGG pathway analyses indicated that all differentially expressed genes included 9 KEGG pathways. The top two pathways were the MAPK signaling pathway and the GnRH signaling pathway. Q-RT-PCR and immunohistochemistry verified that glucagon-like peptide 1 receptor (Glp1r) and mitogen activated protein kinase 10 (Mapk10) were significantly up-regulated, in contrast, gonadotropin releasing hormone receptor (Gnrhr) and gonadotropin-releasing hormone 1 (Gnrh1) were down-regulated in the BerH group.
Conclusion: Our data suggest that berberine can improve blood glucose levels in diabetic rats. The mechanisms involved may be in the MAPK and GnRh-GLP-1 pathways in the ileum.

Berberis vulgaris
Randomised clinical trial of Berberis vulgaris root extract on glycemic and lipid parameters in type 2 diabetes mellitus patients
The main components of barberry are isoquinoline alkaloids such as berberine, berbamine and palmatine [9]. In some studies berberine showed different pharmacological effects [9,12,13]. Berberine is quaternary ammonium salt that is highly concentrated in the roots and stem bark of B. vulgaris [10,14]. Berberine affects glucose metabolism by inhibiting carbohydrate hydrolyzing enzyme and decreasing intestinal glucose absorption [15]. Berberine increases glucagon like peptide-1 (GLP-1) level, therefore decreases pancreatic B cell apoptosis [16]. Some research has indicated that berberine reduces insulin resistance [12].

Bupleurum falcatum
Medicinal Plants Qua Glucagon-Like Peptide-1 Secretagogue via Intestinal Nutrient Sensors
Glucagon-like peptide-1 (GLP-1) participates in glucose homeostasis and feeding behavior. Because GLP-1 is rapidly inactivated by the enzymatic cleavage of dipeptidyl peptidase-4 (DPP4) long-acting GLP-1 analogues, for example, exenatide and DPP4 inhibitors, for example, liraglutide, have been developed as therapeutics for type 2 diabetes mellitus (T2DM). However, the inefficient clinical performance and the incidence of side effects reported on the existing therapeutics for T2DM have led to the development of a novel therapeutic strategy to stimulate endogenous GLP-1 secretion from enteroendocrine L cells. Since the GLP-1 secretion of enteroendocrine L cells depends on the luminal nutrient constituents, the intestinal nutrient sensors involved in GLP-1 secretion have been investigated.
In particular, nutrient sensors for tastants, cannabinoids, and bile acids are able to recognize the nonnutritional chemical compounds, which are abundant in medicinal plants. These GLP-1 secretagogues derived from medicinal plants are easy to find in our surroundings, and their effectiveness has been demonstrated through traditional remedies. The finding of GLP-1 secretagogues is directly linked to understanding of the role of intestinal nutrient sensors and their recognizable nutrients. Concurrently, this study demonstrates the possibility of developing novel therapeutics for metabolic disorders such as T2DM and obesity using nutrients that are readily accessible in our surroundings.

Chebulae Fructus
Tibetan Medicine for Diabetes Mellitus: Overview of Pharmacological Perspectives
Incretins such as GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) are released from the gut, which potentiate insulin release in a glucose-dependent manner and modulate β-cell energetics in intact islets of Langerhans. Recent study showed that the size of islet and the number of β-cell granules stained in deep purple was notably enhanced after 6-weeks of THL administration in STZ-induced diabetic rats, indicating a preservative effect of THL on islet β-cell. THL up-regulated the components of incretin/cAMP signaling pathway including GIP, GLP-1, GLP-1R, and cAMP and promoted the phosphorylation of PKA. It also down-regulated the expression of TXNIP, a vital modulator participating in pancreatic β-cell bioactivity and an indicator of oxidative stress involved in DM progression. This study suggested that THL may enhance the activity of incretin/cAMP signal pathway to affect the proliferation and apoptosis of islet β cells. In addition, supplementation of THL for 45 days obviously reduced the blood glucose levels in non-insulin-dependent diabetic patients

Chlorogenic Acid
Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome
Several human studies have been done to assess the antidiabetic activity of chlorogenic acid-rich foods and supplements, or pure chlorogenic acid. Johnston et al. [48] assessed the antidiabetic effects of dietary amounts of total CGAs in coffee. Nine participants were given 400 mL of water with 25 g of glucose and no coffee, caffeinated coffee (19.96, 10.65, and 10.60 mg/g of chlorogenic acid, 4-CQA, and 3-CQA, respectively), or decaffeinated coffee (11.91, 9.27, 8.26 mg/g of chlorogenic acid, 4-CQA, and 3-CQA, respectively). Both caffeinated and decaffeinated coffees decreased significantly (p < 0.05) the glucose-dependent insulinotropic polypeptide (GIP), suggesting a decrease in the rate of intestinal absorption of glucose. In addition, glucagon-like peptide 1 (GLP-1) secretion significantly increased 0–120 min postprandially after decaffeinated coffee consumption compared to the control. The results suggested that chlorogenic acid might have an antagonistic effect on glucose transport.
A chlorogenic acid-induced increase in GLP-1 production may mediate the impact of heavy coffee consumption on diabetes risk
Recent prospective epidemiology links heavy coffee consumption to a substantial reduction in risk for type 2 diabetes. Yet there is no evidence that coffee improves insulin sensitivity and, at least in acute studies, caffeine has a negative impact in this regard. Thus, it is reasonable to suspect that coffee influences the risk for beta cell “failure” that precipitates diabetes in subjects who are already insulin resistant. Indeed, there is recent evidence that coffee increases production of the incretin hormone glucagon-like peptide-1 (GLP-1), possibly owing to an inhibitory effect of chlorogenic acid (CGA — the chief polyphenol in coffee) on glucose absorption. GLP-1 acts on beta cells, via cAMP-dependent mechanisms, to promote the synthesis and activity of the transcription factor IDX-1, crucial for maintaining the responsiveness of beta cells to an increase in plasma glucose. Conversely, the “glucolipotoxicity” thought to initiate and sustain beta cell dysfunction in diabetics can suppress expression of this transcription factor.
The increased production of GLP-1 associated with frequent coffee consumption could thus be expected to counteract the adverse impact of chronic free fatty acid overexposure on beta cell function in overweight insulin resistant subjects. CGA’s putative impact on glucose absorption may reflect the ability of this compound to inhibit glucose-6-phosphate translocase 1, now known to play a role in intestinal glucose transport. Delayed glucose absorption may itself protect beta cells by limiting postprandial hyperglycemia — though, owing to countervailing effects of caffeine on plasma glucose, and a paucity of relevant research studies, it is still unclear whether coffee ingestion blunts the postprandial rise in plasma glucose. More generally, diets high in “lente carbohydrate”, or administration of nutraceuticals/pharmaceuticals which slow the absorption of dietary carbohydrate, should help preserve efficient beta cell function by boosting GLP-1 production, as well as by blunting the glucotoxic impact of postprandial hyperglycemia on beta cell function.

Cinnamomi Cortex
Cinnamon Dose-Dependently Reduces Insulin Concentration
The ingestion of 3 g of cinnamon reduces serum insulin levels after mealtime and increases the concentration of glucagon-like peptide 1 (GLP-1), a gastrointestinal hormone that has been shown to delay gastric emptying and minimize the feeling of hunger after eating, according to a study published in the March 1 issue of the American Journal of Clinical Nutrition. There was no significant affect on blood glucose, glucose-dependent insulinotropic polypeptide (GIP), ghrelin concentration, satiety, or gastric emptying rate (GER). Furthermore, there appeared to be an inverse relationship between the amount of cinnamon consumed and the reduction in insulin concentration.
Previously, the ingestion of 6 g of cinnamon was shown to be associated with a reduction in blood glucose concentrations after mealtime, as well as GER, without affecting satiety among a group of healthy subjects. “Six grams of cinnamon is not a quantity ordinarily used in food,” explains Joanna Hlebowicz, MD, from the Department of Medicine, Malmo University Hospital, Sweden, and colleagues. “[Thus,] the primary objective of this study was to determine whether adding 3 g cinnamon to a meal would change GER, satiety, or postprandial blood glucose, insulin, GIP, and GLP-1 concentrations. If this was the case, the secondary objective was to establish whether adding 1 g cinnamon to a meal would change GER, satiety, and postprandial blood glucose, insulin, GIP, and GLP-1 concentrations.”
In a crossover study, a total of 15 healthy Swedish men (n = 9) and women (n = 6) were given 300 g of rice pudding with 3 g, 1 g, or no cinnamon after a period of fasting. Meals were served in random order at weekly intervals. Using real-time ultrasonography, GER was measured in each participant after meals. A scoring scale was used to determine satiety among the subjects before and after eating at prespecified intervals. In addition, venous blood samples were evaluated for the measurement of glucose, insulin, GIP, GLP-1, and ghrelin concentrations both before and after each mealtime according to the same prespecified intervals.
“This study was…designed to determine whether changes in insulin response explain lower postprandial blood glucose concentrations and whether a change in GIP, GLP-1, or ghrelin response could explain the change in insulin secretion or the change in GER in healthy subjects after cinnamon consumption,” write the authors. “We also studied the dose-response relation with respect to the effect on GER, satiety, and postprandial blood glucose, insulin, GIP, GLP-1, and ghrelin responses in healthy subjects.”
The results illustrated a significant reduction following the consumption of rice pudding with 3 g of cinnamon in the insulin response at 60 minutes (P = .05, after Bonferroni correction), as well as the area under the curve at 120 minutes (P = .036, after Bonferroni correction). Similarly, there was a significant increase following the consumption of rice pudding with 3 g of cinnamon in the GLP-1 response (P = .0082, after Bonferroni correction) and the change in the maximum concentration (P = .0138, after Bonferroni correction). However, there was no significant difference in GER, satiety, glucose, GIP, or the ghrelin response following the ingestion of the rice pudding with 3 g, 1 g, or no cinnamon.
cinnamtannin A2
Cinnamtannin A2, a Tetrameric Procyanidin, Increases GLP-1 and Insulin Secretion in Mice
Procyanidins are oligomers and polymers of flavan-3-ols consisting of (−)-epicatechin subunits. In this study, we isolated and purified dimeric, trimeric and tetrameric procyanidins from cacao liquor and investigated their influence on the “incretin effect” as compared to the monomer, (−)-epicatechin in mice. Cinnamtannin A2 specifically increased the glucagon-like peptide-1 (GLP-1) and insulin secretion levels in the plasma after 60 min administration. As evidence of the action of insulin, activation of insulin receptor and insulin receptor substrate-1 was observed in the soleus muscle. These results indicate that the intake of cinnamtannin A2 may improve hyperglycemia through an incretin-like effect, accompanied by activation of the insulin-signaling pathway.

Citrus aurantium
Hexane fraction of Citrus aurantium L. stimulates glucagon-like peptide-1 (GLP-1) secretion via membrane depolarization in NCI-H716 cells
Citrus species have been used traditionally as a medicinal herb in oriental pharmacology. Here, we reported on the anti-diabetic function of Citrus aurantium L. (CA). The hexane fraction of CA (HFCA) stimulates NCI-H716 cells and results in the secretion of glucagon-like peptide-1 (GLP-1). Because it regulates insulin secretion in pancreatic β-cells, GLP-1 has been used for the treatment of type II diabetes mellitus. Hence, we carried out a series of experiments to demonstrate the functions of HFCA against diabetes mellitus at the molecular level.
Four fractions of CA were used in a GLP-1 assay. The hexane fraction showed the best results and was chosen for the microarray analysis in the genome wide analysis. Through the analysis, it was found that voltage-gated potassium (Kv) channels drove membrane depolarization and then influenced Ca2+ currents in NCIH716 cells. These results suggest this is a new oriental herbal drug that has proven effects for the remedy of type II diabetes mellitus.

Curcumin
Boosting GLP-1 by Natural Products
The prevalence of diabetes mellitus is growing rapidly. Diabetes is the underlying cause of many metabolic and tissue dysfunctions, and, therefore, many therapeutic agents have been developed to regulate the glycemic profile. Glucagon-like peptide-1 (GLP-1) receptor agonists are a newly developed class of antidiabetic drugs that have potent hypoglycemic effects via several molecular pathways. In addition to synthetic GLP-1 receptor agonists, some evidence suggests that natural products may have modulatory effects on GLP-1 expression and secretion. In the current study, we conclude that certain herbal-based constituents, such as berberine, tea, curcumin, cinnamon, wheat, soybean, resveratrol, and gardenia, can exert an influence on GLP-1 release.

Cynanchum marnierianum
Pregnane Glycosides from Cynanchum marnierianum Stimulate GLP-1 Secretion in STC-1 Cells
In the framework of the search for natural glucagon-like peptide-1 secretagogues, the bioassay-guided fractionation of the ethanolic extract from Cynanchum marnierianum led to the isolation of two new pregnane glycosides named marnieranosides A (1) and B (2). The structures were determined based on spectroscopic data and were established as 12β,20 S-O-dibenzoyl-pregn-6-en-5α,8β,14β,17β-tetraol-3-O-β-D-oleandropyranosyl-(1 → 4)-β-D-cymaropyranoside (1) and 12β,20R-O-dibenzoyl-pregn-6-en-5α,8β,14β-triol-3-O-β-D-oleandropyranosyl-(1 → 4)-β-D-canaropyranosyl-(1 → 4)-β-D-cymaropyranoside (2). They present structural analogies to pregnanes previously described in species known for their appetite suppressant and antihyperglycemic effects, such as P57 from Hoodia gordonii. Lupeol (3), a known dipeptidyl peptidase-4 inhibitor, and the insulinomimetic kaempferol-3-O-neohesperidoside (4) were also identified in C. marnierianum.
In an in vitro assay on secretin tumor cell line-1 cells, compounds 1, 2, and P57 were found to stimulate the secretion of GLP-1 by 130 % (all tested at 100 µM). These results suggest that C. marnierianum could be of great interest in the treatment of type 2 diabetes, and that pregnane derivatives should be partly responsible via the stimulation of glucagon-like peptide-1 secretion.

Delphinidin
Delphinidin 3-rutinoside-rich blackcurrant extract ameliorates glucose tolerance by increasing the release of glucagon-like peptide-1 secretion
Glucagon-like peptide-1 (GLP-1) is released from enteroendocrine cells (L cells) in response to food ingestion. The mechanism by which dietary peptides stimulate GLP-1 secretion in the gut is unknown. In the present study, we found that a hydrolysate prepared from zein, a major corn protein [zein hydrolysate (ZeinH)], strongly stimulates GLP-1 secretion in enteroendocrine GLUTag cells. Stimulatory mechanisms of GLP-1 secretion induced by ZeinH were investigated in the rat small intestine under anesthesia. Blood was collected through a portal catheter before and after ZeinH administration into different sites of the small intestine. The duodenal, jejunal, and ileal administration of ZeinH induced dose-dependent increases in portal GLP-1 concentration. GLP-1 secretion in response to the ileal administration of ZeinH was higher than that in the duodenal or jejunal administration. Capsaicin treatment on esophageal vagal trunks abolished the GLP-1 secretion induced by duodenal ZeinH but did not affect the secretion induced by jejunal or ileal ZeinH.
These results suggest that ZeinH in the jejunum or ileum directly stimulates GLP-1 secretion but duodenal ZeinH indirectly stimulates GLP-1 secretion via the vagal afferent nerve. A direct blood sampling method from the duodenal vein and ileal mesenteric vein revealed that ZeinH administered into the ligated duodenal loop enhanced GLP-1 concentration in the ileal mesenteric vein but not in the duodenal vein. This confirmed that ZeinH in the duodenum induces GLP-1 secretion from L cells located in the ileum by an indirect mechanism. These results indicate that a potent GLP-1-releasing peptide, ZeinH, induces GLP-1 secretion by direct and indirect mechanisms in the rat intestine.

Dendrobii Officmalis
Structural characterization and hypoglycemic effect via stimulating glucagon-like peptide-1 secretion of two polysaccharides from Dendrobium officinale
Two polysaccharides, named DOP-1 and DOP-2, with molecular weights of 6.8 kDa and 14.3 kDa, respectively, were isolated and purified from the stems of Dendrobium officinale. Monosaccharide composition, Fourier-transform infrared spectroscopy, methylation, and nuclear magnetic resonance analyses indicated that DOP-1 and DOP-2 may have a backbone consisted of →4)-β-d-Glcp-(1→, →4)-β-d-Manp-(1→, →4)-2-O-acetyl-β-d-Manp-(1→ and →4)-3-O-acetyl-β-d-Manp-(1→. In vivo assays showed that D. officinale polysaccharides (DOPs) exerted significant hypoglycemic effects accompanying increased serum insulin and glucagon-like peptide-1 (GLP-1) levels in streptozotocin-induced diabetic rats.
Further in vitro experiments showed that DOP-induced GLP-1 secretion was inhibited by an intracellular calcium chelator, a Ca2+/calmodulin-dependent protein kinase (CaMK) II inhibitor, a specific calcium-sensing receptor antagonist, and a p38-mitogen-activated protein kinases (MAPK) inhibitor. These results indicated that DOPs may decrease fasting blood sugar levels by stimulating GLP-1 secretion and that intracellular DOP-induced GLP-1 secretion involved the Ca2+/calmodulin/CaMKII and MAPK pathways.
Physicochemical property changes of Dendrobium officinale leaf polysaccharide LDOP-A and it promotes GLP-1 secretion in NCI-H716 cells by simulated saliva-gastrointestinal digestion
The available evidence suggests that polysaccharides can slow down or reverse the decrease of butyric-acid-producing bacteria and intestinal butyric acid levels in T2DM patients (Kumar et al., 2021; Qin et al., 2021). Some studies have shown that butyric acid can increase the secretion of Glucagon-like peptide-1 (GLP-1) and peptide YY in the intestine, thus promoting the secretion of insulin in T2DM patients (Fan & Pedersen, 2020). GLP-1 is a kind of incretin secreted under the stimulation of eating, which is released by L cells of the distal ileum and colon (Pan et al., 2021). Previous studies have indicated that plant polysaccharides have an excellent hypoglycemic effect by increasing the levels of insulin and GLP-1 in streptozotocin-induced diabetic rats (Lee et al., 2018). GLP-1 can control the fluctuation of blood sugar by increasing insulin secretion, reducing glucagon level and appetite, and also can promote pancreatic islets β-cell proliferation, differentiation, and reduction of β apoptosis (Kuang et al., 2020; Xie et al., 2020; Yang et al., 2021).
In order to detect the in vitro anti-diabetes effect of the polysaccharide extracted from Dendrobium officinale leaf named LDOP-A, the present study investigated the structure and simulated digestion of LDOP-A in vitro and then explored secretion of GLP-1 from endocrine L cells by direct stimulation of digested LDOP-A.
A polysaccharide LDOP-A with a molecular weight of 9.9 kDa was isolated and purified from Dendrobium officinale leaves by membrane separation, cellulose column, and dextran gel column. The Smith degradable products, methylation products, and nuclear magnetic resonance analysis showed that LDOP-A may be composed of →4)-Glc-(1→, →3,6)-Man-(1→, and →6)-Glc-(1→sugar residues. In vitro, simulated digestion assays showed that LDOP-A could be partially digested in the stomach and small intestine, and produced a large amount of acetic acid and butyric acid during colonic fermentation. Further cell experiment results illustrated that LDOP-A-I (LDOP-A digested by gastrointestinal tract) could induce glucagon-like peptide-1 (GLP-1) secretion in NCI-H716 cells without showing any cytotoxicity.

EGCG
The combined administration of EGCG and caffeine induces not only suppression of fat accumulation but also anorexigenic action in mice
To elucidate the anorexigenic action and inhibitory effect of fat accumulation by epigallocatechin gallate (EGCG) and caffeine, including the optimal combination ratio and mechanism, fifteen diets with several concentrations of EGCG and/or caffeine were administered to mice for eight weeks. The 0.1% EGCG + 0.1% caffeine group showed the strongest suppression of food intake and a remarkable reduction of body weight and fat accumulation; therefore, the ratio was determined to be the optimal combination ratio. Moreover, serum glucagon-like peptide-1 (GLP-1) level and hypothalamic gene expression of pro-opiomelanocortin (POMC) were promoted by 0.1% EGCG + 0.1% caffeine. In conclusion, the combined treatment of 0.1% EGCG + 0.1% caffeine induces not only suppression of fat accumulation but also strong anorexigenic action in mice. The anorexigenic effect may be brought about via inhibiting gastric motility by GLP-1 and upregulating POMC in the hypothalamus.

Elaterin
Role of GLP-1 in the Hypoglycemic Effects of Wild Bitter Gourd
This study aimed to examine the role of GLP-1 in the hypoglycemic activity of wild bitter gourd (Momordica charantia L., BG). In vitro, the GLP-1 secretion in STC-1, a murine enteroendocrine cell line, was dose dependently stimulated by water extract (WE), its fractions (WEL, >3 kD and WES, <3 kD), and a bitter compounds-rich fraction of BG. These stimulations were partially inhibited by probenecid, a bitter taste receptor inhibitor, and by U-73122, a phospholipase Cβ2 inhibitor.
These results suggested that the stimulation might involve, at least in part, certain bitter taste receptors and/or PLCβ2-signaling pathway. Two cucurbitane triterpenoids isolated from BG, 19-nor-cucurbita-5(10),6,8,22-(E),24-pentaen-3β-ol, and 5β,19-epoxycucurbita-6,24-diene-3β,23ξ-diol (karavilagenine E,) showed relative high efficacy in the stimulation. In vivo, mice fed BG diet showed higher insulinogenic index in an oral glucose tolerance test. A single oral dose of WE or WES pretreatment significantly improved intraperitoneal glucose tolerance. A single oral dose of WES significantly decreased glucose and increased insulin and GLP-1 in serum after 30 min. This acute hypoglycemic effect of WES was abolished by pretreatment with exendin-9, a GLP-1 receptor antagonist. Our data provide evidence that BG stimulates GLP-1 secretion which contributes, at least in part, to the antidiabetic activity of BG through an incretin effect.
Potential use of bitter melon (Momordica charantia) derived compounds as antidiabetics: In silico and in vivo studies
Momordica charantia (bitter lemon) belongs to the cucurbitaceae family which has been extensively used in traditional medicines for the cure of various ailments such as cancer and diabetes. The underlying mechanism of M. charantia to maintain glycemic control was investigated. GLP-1 and DPP-4 gene modulation by M. charantia (5–20% inclusion in rats diet) was investigated in vivo by RT-PCR and possible compounds responsible for diabetic action predicted through in silico approach. Phytochemicalss previously characterized from M. charantia were docked into glucacon like peptide-1 receptor (GLP-1 R), dipeptidyl peptidase (DPP4) and Takeda-G-protein-receptor-5 (TGR5) predicted using Autodock Vina.
The results of the in silico suggests momordicosides D (ligand for TGR5), cucurbitacin (ligand for GLP-1 R) and charantin (ligand for DPP-4) as the major antidiabetic compounds in bitter lemon leaf. M. charantia increased the expression of GLP-1 by about 295.7% with concomitant decreased in expression of DPP-4 by 87.2% with 20% inclusion in rat’s diet. This study suggests that the mechanism underlying the action of these compounds is through activation of TGR5 and GLP-1 receptor with concurrent inhibition of DPP4. This study confirmed the use of this plant in diabetes management and the possible bioactive compounds responsible for its antidiabetic property are charantin, cucurbitacin and momordicoside D and all belong to the class of saponins.

Eucalyptus citriodora
Insulin Secretory Actions of Ethanol Extract of Eucalyptus citriodora Leaf, including Plasma DPP-IV and GLP-1 Levels in High-Fat-Fed Rats, as Well as Characterization of Biologically Effective Phytoconstituents
Due to the numerous adverse effects of synthetic drugs, researchers are currently studying traditional medicinal plants to find alternatives for diabetes treatment. Eucalyptus citriodora is known to be used as a remedy for various illnesses, including diabetes. This study aimed to explore the effects of ethanol extract of Eucalyptus citriodora (EEEC) on in vitro and in vivo systems, including the mechanism/s of action. The methodology used involved the measurement of insulin secretion from clonal pancreatic β-cells, BRIN BD11, and mouse islets. Other in vitro systems further examined EEEC’s glucose-lowering properties. Obese rats fed a high-fat diet (HFF) were selected for in vivo evaluation, and phytoconstituents were detected via RP-HPLC followed by LC-MS. EEEC induced insulin secretion in a concentration-dependent manner with modulatory effects, similar to 1 µM glucagon-like peptide 1 (GLP-1), which were partly declined in the presence of Ca2+-channel blocker (Verapamil), KATP-channel opener (Diazoxide), and Ca2+ chelation.
The insulin secretory effects of EEEC were augmented by isobutyl methylxanthine (IBMX), which persisted in the context of tolbutamide or a depolarizing concentration of KCl. EEEC enhanced insulin action in 3T3-L1 cells and reduced glucose absorption, and protein glycation in vitro. In HFF rats, it improved glucose tolerance and plasma insulin, attenuated plasma DPP-IV, and induced active GLP-1 (7-36) levels in circulation. Rhodomyrtosone B, Quercetin-3-O-β-D-glucopyranoside, rhodomyrtosone E, and quercitroside were identified as possible phytoconstituents that may be responsible for EEEC effects. Thus, these findings revealed that E. citriodora could be used as an adjunct nutritional supplement to manage type 2 diabetes.

Fagopyrum tataricum
Dietary supplementation of rutin and rutin-rich buckwheat elevates endogenous glucagon-like peptide 1 levels to facilitate glycemic control in type 2 diabetic mice
Rutin and rutin-rich buckwheat are commonly used as alternative medicines due to their wide range of health benefits. The present study aims to investigate the anti-diabetic effects of rutin and buckwheat on insulin resistance, oxidative stress, and GLP-1 in type 2 diabetes (T2D) rats.
In this study, 40 male Wistar male rats were divided into 5 groups: 1. Control group, 2. T2D, 3. T2D treated with probiotics, 4. T2D treated with resveratrol, 5. T2D group treated with probiotics and resveratrol. After four weeks, the intestine were removed for histopathological analysis, biochemical tests, and oxidative stress markers.
Results: Probiotics and resveratrol significantly decreased (p < 0.001) glucose and insulin resistance, and increased (p < 0.001) GLP-1 and total antioxidant capacity compared to the diabetic group. Treatment with probiotics and resveratrol also returned intestinal histological changes in diabetic rats to normal.
Conclusion: Resveratrol and probiotics appear to be effective in controlling T2D by increasing GLP-1 levels and reducing oxidative stress.

Fructus Gardeniae
Effect of intake of gardenia fruits and combined exercise of middle-aged obese women on hormones regulating energy metabolism
According to the results of this study, the gardenia + exercise group, the exercise group, and the gardenia group showed a decrease in GLP-1, which was consistent with the previous findings [13,21,37]. Considering the previous studies, a decreased GLP-1 secretin in the exercise group was ascribed to an increased energy consumption resulting from exercise and weight loss due to appetite suppression, and the subsequent negative balance contributed to decreasing GLP-1. The cause of a decrease in the GLP-1 of the gardenia + exercise group and the gardenia group is because the gardenia may contains an appetite suppressing ingredient, which led to a reduced energy intake.
Thus, the obtained conclusion is as follows: The %fat and WHR has decreased further in the gardenia+exercise group and the exercise group as compared with the control group. And the visceral fat area has decreased further in the gardenia and exercise group and the gardenia group as compared with the control group (p<.05). In addition, the gardenia+exercise group and the exercise group were found to have a significant improvement effect in all the items of body composition, and the gardenia group has reduced the fat percentage and BMI after the treatment (p<.05). Leptin has decreased further in the gardenia+exercise group and the exercise group as compared with the control group, and the insulin resistance and GLP-1 have decreased in all the treatment groups (p<.05).

Ganoderma lucidum
Mushrooms of the Genus Ganoderma Used to Treat Diabetes and Insulin Resistance
Incretin therapies include, in addition to DPP-4 inhibitors, also subcutaneously injectable glucagon-like peptide 1 (GLP-1) receptor agonists (e.g., exenatide, liraglutide, lixisenatide) [10]. GLP-1 agonists are parenteral agents that mimic actions of endogenous glucagon-like peptide-1. They lower plasma glucose levels by several mechanisms, including inhibition of glucagon secretion and enhancement of insulin release in a glucose-dependent manner [11]. The last group of antidiabetic drugs are sodium-glucose cotransporter 2 (SGLT 2) inhibitors such as canagliflozin, dapagliflozin, and empagliflozin. Some of the most common side effects of SGLT 2 inhibitors is urogenital tract infection, genital infection, or even breast and bladder cancer [4].

Gardenia jasminoides
Boosting GLP-1 by Natural Products
The prevalence of diabetes mellitus is growing rapidly. Diabetes is the underlying cause of many metabolic and tissue dysfunctions, and, therefore, many therapeutic agents have been developed to regulate the glycemic profile. Glucagon-like peptide-1 (GLP-1) receptor agonists are a newly developed class of antidiabetic drugs that have potent hypoglycemic effects via several molecular pathways. In addition to synthetic GLP-1 receptor agonists, some evidence suggests that natural products may have modulatory effects on GLP-1 expression and secretion. In the current study, we conclude that certain herbal-based constituents, such as berberine, tea, curcumin, cinnamon, wheat, soybean, resveratrol, and gardenia, can exert an influence on GLP-1 release.

Genistein
Genistein enhances the secretion of glucagon-like peptide-1 (GLP-1) via downregulation of inflammatory responses
Glucagon-like peptide-1 (GLP-1) an incretin hormone, is known to regulate the glucose-mediated insulin secretion. However, reduction in the level of GLP-1 is considered to be a major cause for the reduction of GLP-1-dependent insulin secretory response. Genistein an isoflavone, is an important polyphenol and has wide range of therapeutic potentials, but its therapeutic effects alone and/or in combination with metformin on GLP-1 secretion have not been investigated yet. Hence, we aimed to investigate the stimulatory action of genistein in combination with metformin on GLP-1 via downregulation of inflammatory mediators, hyperlipidemia and hyperglycemia in alloxan-induced diabetic rats. Diabetes was induced in experimental rats by single administration of alloxan intraperitoneally. Metformin (50 mg/kg/day), genistein (20 mg/kg/day) and combination of genistein and metformin was administered in alloxan-induced diabetic rats. We found that genistein alone and/or in combination with metformin significantly increased the serum level (P < 0.01) and tissue content (P < 0.05) of GLP-1 in intestine when compared with that of metformin-treated animals. Similarly, genistein alone and/or in combination with metformin also resulted in normoglycemia (P < 0.001), glucose tolerance (P < 0.01), insulin sensitivity (P < 0.0001), hyperlipidemia (P < 0.01), liver and kidney function biomarkers (P < 0.01) as compared to that of metformin-treated experimental animals.
Moreover, genistein alone and/or in combination with metformin also downregulated the inflammatory responses by decreasing the levels of interleuin-6, tumor necrosis factor-α and C-reactive protein in serum (P < 0.05) and intestine (P < 0.001) more efficiently as compared to that of metformin-treated experimental animals. The downregulation of inflammatory responses in intestine, was positively associated with increased secretion of GLP-1 from intestine. Histopathology of pancreas and intestine also showed that genistein significantly improved the deleterious effects of alloxan on pancreas and intestine. Hence, our work provides new insights on the synergistic effects of genistein and metformin on GLP-1 secretion. This may significantly improve the perception for proposing new GLP-1-based synergistic approaches for the treatment of diabetes mellitus.

Gentiana scabra
Medicinal Plants Qua Glucagon-Like Peptide-1 Secretagogue via Intestinal Nutrient Sensors
Glucagon-like peptide-1 (GLP-1) participates in glucose homeostasis and feeding behavior. Because GLP-1 is rapidly inactivated by the enzymatic cleavage of dipeptidyl peptidase-4 (DPP4) long-acting GLP-1 analogues, for example, exenatide and DPP4 inhibitors, for example, liraglutide, have been developed as therapeutics for type 2 diabetes mellitus (T2DM). However, the inefficient clinical performance and the incidence of side effects reported on the existing therapeutics for T2DM have led to the development of a novel therapeutic strategy to stimulate endogenous GLP-1 secretion from enteroendocrine L cells. Since the GLP-1 secretion of enteroendocrine L cells depends on the luminal nutrient constituents, the intestinal nutrient sensors involved in GLP-1 secretion have been investigated.
In particular, nutrient sensors for tastants, cannabinoids, and bile acids are able to recognize the nonnutritional chemical compounds, which are abundant in medicinal plants. These GLP-1 secretagogues derived from medicinal plants are easy to find in our surroundings, and their effectiveness has been demonstrated through traditional remedies. The finding of GLP-1 secretagogues is directly linked to understanding of the role of intestinal nutrient sensors and their recognizable nutrients. Concurrently, this study demonstrates the possibility of developing novel therapeutics for metabolic disorders such as T2DM and obesity using nutrients that are readily accessible in our surroundings.
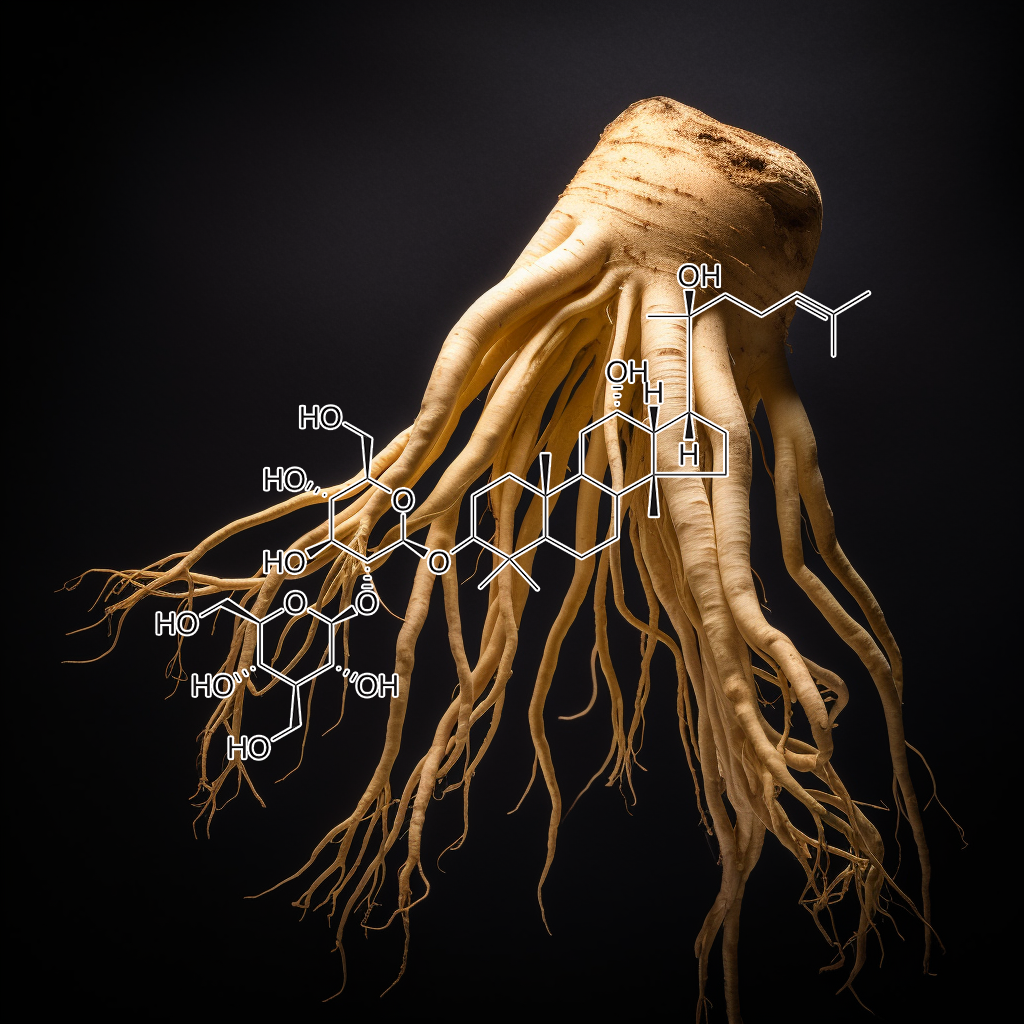
Ginsenoside Rb1 Rg1 Rg5 T19 Rk3
Increased glucagon-like peptide-1 secretion may be involved in antidiabetic effects of ginsenosides
Panax ginseng is one of the most popular herbal remedies. Ginsenosides, major bioactive constituents in P. ginseng, have shown good antidiabetic action, but the precise mechanism was not fully understood. Glucagon-like peptide-1 (GLP1) is considered to be an important incretin that can regulate glucose homeostasis in the gastrointestinal tract after meals. The aim of this study was to investigate whether ginseng total saponins (GTS) exerts its antidiabetic effects via modulating GLP1 release. Ginsenoside Rb1 (Rb1), the most abundant constituent in GTS, was selected to further explore the underlying mechanisms in cultured NCI-H716 cells. Diabetic rats were developed by a combination of high-fat diet and low-dose streptozotocin injection. The diabetic rats orally received GTS (150 or 300 mg/kg) daily for 4 weeks. It was found that GTS treatment significantly ameliorated hyperglycemia and dyslipidemia, accompanied by a significant increase in glucose-induced GLP1 secretion and upregulation of proglucagon gene expression. Data from NCI-H716 cells showed that both GTS and Rb1 promoted GLP1 secretion. It was observed that Rb1 increased the ratio of intracellular ATP to ADP concentration and intracellular Ca2+ concentration. The metabolic inhibitor azide (3 mM), the KATP channel opener diazoxide (340 μM), and the Ca2+ channel blocker nifedipine (20 μM) significantly reversed Rb1-mediated GLP1 secretion. All these results drew a conclusion that ginsenosides stimulated GLP1 secretion both in vivo and in vitro. The antidiabetic effects of ginsenosides may be a result of enhanced GLP1 secretion.
Review of Ginseng Anti-Diabetic Studies
Ginsenoside Rb1, one of the main PPD-type ginsenosides, was found to reduce symptoms of decreased insulin sensitivity and elevated blood glucose caused by the high-fat diet induction of type 2 diabetic mice [31]. Using the STZ-induced diabetic rat model, researchers found that a main PPT-type ginsenoside, Rg1, can lower insulin resistance and blood glucose, and also improve the blood lipid profile and liver function [32], suggesting that Rg1 may be a potential adjuvant therapy for type 2 diabetic patients with fatty liver disease. As well as the main components in fresh ginseng extracts, heat-treated ginsenoside Rg3 has also been tested.
Ginsenoside Rg3 is the main metabolite degraded from other abundant ginsenosides during heat-processing fresh ginseng to manufacture red ginseng and black ginseng. Kim et al. screened the GLP-1 release ability of 15 ginsenosides and found that Rg3 showed the strongest GLP-1 secretion (about 27 pM/mg) effect in NCI-H716 cells. In this in vivo trial, 10 µM Rg3 significantly raised production of GLP-1 and insulin to reduce blood glucose in db/db mice [33]. In mouse islet cells, insulin secretion was significant: 2.3 times higher in the 4 μM Rg3 treatment group compared to control group [34]. Taken together, Rg3 is presumably the main active anti-diabetic ingredient of ginseng, although administration with other ginsenosides has also shown hypoglycemic effects; these ginsenosides can be hydrolysed to Rg3 in the gastrointestinal tract [35] to further exert pharmacological effects.

Glycine Max
Black soybean seed coat polyphenols promote nitric oxide production in the aorta through glucagon-like peptide-1 secretion from the intestinal cells
The administration of black soybean seed coat extract (BE) also increased GLP-1 and cAMP levels. Furthermore, the effects of BE were inhibited in the presence of a GLP-1 receptor antagonist. This suggests that GLP-1 is strongly involved in the underlying mechanism of NO production in vivo. In conclusion, BE contributes to the improvement of vascular functions by promoting NO production. Regarding the putative underlying mechanism, GLP-1 secreted from intestinal cells by the polyphenols in BE activates eNOS in vascular endothelial cells.

Grifolic Acid
The Emerging Role of Polyphenols in the Management of Type 2 Diabetes – PMC
Type 2 diabetes (T2D) is a fast-increasing health problem globally, and it results from insulin resistance and pancreatic β-cell dysfunction. The gastrointestinal (GI) tract is recognized as one of the major regulatory organs of glucose homeostasis that involves multiple gut hormones and microbiota. Notably, the incretin hormone glucagon-like peptide-1 (GLP-1) secreted from enteroendocrine L-cells plays a pivotal role in maintaining glycemic homeostasis via eliciting pleiotropic effects, which are largely mediated via its receptor. Thus, targeting the GLP-1 signaling system is a highly attractive therapeutic strategy to treatment T2D. Polyphenols, the secondary metabolites from plants, have drawn considerable attention because of their numerous health benefits, including potential anti-diabetic effects.
Although the major targets and locations for the polyphenolic compounds to exert the anti-diabetic action are still unclear, the first organ that is exposed to these compounds is the GI tract in which polyphenols could modulate enzymes and hormones. Indeed, emerging evidence has shown that polyphenols can stimulate GLP-1 secretion, indicating that these natural compounds might exert metabolic action at least partially mediated by GLP-1. This review provides an overview of nutritional regulation of GLP-1 secretion and summarizes recent studies on the roles of polyphenols in GLP-1 secretion and degradation as it relates to metabolic homeostasis. In addition, the effects of polyphenols on microbiota and microbial metabolites that could indirectly modulate GLP-1 secretion are also discussed.
Free Fatty Acid Receptor GPR120 Is Highly Expressed in Enteroendocrine K Cells of the Upper Small Intestine and Has a Critical Role in GIP Secretion After Fat Ingestion | Endocrinology | Oxford Academic
Gastric inhibitory polypeptide (GIP) is an incretin secreted from enteroendocrine K cells in response to meal ingestion. Recently free fatty acid receptor G protein-coupled receptor (GPR) 120 was identified as a lipid sensor involved in glucagon-like peptide-1 secretion. However, Gpr 120 gene expression and its role in K cells remain unclear, partly due to difficulties in separation of K cells from other intestinal epithelial cells. In this study, we purified K cells using GIP-green fluorescent protein (GFP) knock-in mice, in which K cells can be visualized by GFP fluorescence. GFP-positive cells (K cells) were observed in the small intestine but not in the stomach and colon. K cell number and GIP content in K cells were significantly higher in the upper small intestine than those in the lower small intestine. We also examined the expression levels of several free fatty acid receptors in K cells.
Among free fatty acid receptors, GPR120 was highly expressed in the K cells of the upper small intestine compared with the lower small intestine. To clarify the role of GPR120 on K cells in vivo, we used GPR120-deficient mice (GPR120−/−). GPR120−/−exhibited significantly lower GIP secretion (75% reduction, P < .01) after lard oil ingestion compared with that in wild-type mice. Consistently, pharmacological inhibition of GPR120 with grifolic acid methyl ether in wild-type mice significantly attenuated lard oil-induced GIP secretion. In conclusion, GPR120 is expressed abundantly in K cells of the upper small intestine and plays a critical role in lipid-induced GIP secretion.

Hispidulin
Flavone Hispidulin Stimulates Glucagon-Like Peptide-1 Secretion and Ameliorates Hyperglycemia in Streptozotocin-Induced Diabetic Mice
Loss of functional β-cell mass is central for the deterioration of glycemic control in diabetes. The incretin hormone glucagon-like peptide-1 (GLP-1) plays a critical role in maintaining glycemic homeostasis via potentiating glucose-stimulated insulin secretion and promoting β-cell mass. Agents that can directly promote GLP-1 secretion, thereby increasing insulin secretion and preserving β-cell mass, hold great potential for the treatment of T2D.
GluTag L-cells, INS832/13 cells, and mouse ileum crypts and islets are cultured for examining the effects of flavone hispidulin on GLP-1 and insulin secretion. Mouse livers and isolated hepatocytes are used for gluconeogenesis. Streptozotocin-induced diabetic mice are treated with hispidulin (20 mg kg−1 day−1, oral gavage) for 6 weeks to evaluate its anti-diabetic potential. Hispidulin stimulates GLP-1 secretion from the L-cell line, ileum crypts, and in vivo. This hispidulin action is mediated via activation of cyclic adenosine monophosphate/protein kinase A signaling. Hispidulin significantly improves glycemic control in diabetic mice, concomitant with improved insulin release, and β-cell survival. Additionally, hispidulin decreases hepatic pyruvate carboxylase expression in diabetic mice and suppresses gluconeogenesis in hepatocytes. Furthermore, hispidulin stimulates insulin secretion from β-cells.
These findings suggest that Hispidulin may be a novel dual-action anti-diabetic compound via stimulating GLP-1 secretion and suppressing hepatic glucose production.

Hoodia Gordonii
Molecular matchmaking between the popular weight-loss herb Hoodia gordonii and GPR119, a potential drug target for metabolic disorder
In the present study, we discovered that Gordonoside F, a pregnane steroidal glycoside isolated from H. gordonii (8), is a novel and highly selective agonist of GPR119. Interestingly, the natural congeners (1, 2, and 4–6) of Gordonoside F occurring in the same plant, including P57 (1), displayed no such activity. GPR119 is expressed predominantly in pancreatic β cells and in enteroendocrine cells. It has become a major target for the development of antidiabetic/obesity drugs because GPR119 activation can stimulate both insulin release from the pancreas and GLP-1 release from the intestine (9, 24, 25). GLP-1 is a potent insulin-releasing and appetite-suppressing hormone, and GLP-1 analogs have been used to treat type 2 diabetes in clinics (26, 27). GPR119 is activated predominantly by fatty acid ethanolamide derivatives, the phospholipid lysophosphatidylcholine (28), and 5-hydroxy eicosapentanoic acid (5-HEPE) (29).

Ilex Paraguariensis
The Positive Effects of Yerba Maté (Ilex paraguariensis) in Obesity
In addition to human studies, in DdY mice fed with high-fat diet animal models, yerba maté has been suggested to promote satiety through various mechanisms, including induction and/or enhancement of intestinal glucagon-like peptide-1 (GLP-1), modulation of serum leptin levels and a possible direct central satiety-stimulatory effect [61]. Data obtained from experiments conducted in diet-induced obesity models have shown that yerba maté suppresses body weight gain and visceral fat accumulation and decreases serum levels of cholesterol, triglycerides, LDL cholesterol, glucose, insulin, pancreatic lipase and leptin [39,44,47,48,49,56,58,59]. Additionally, yerba maté reduces endothelin and thromboxane A2 levels and increases nitric oxide and 6-keto-PGF1α levels in the blood, inhibiting the occurrence of atherosclerosis [69]. It has been suggested that the high polyphenol content of yerba maté might be responsible for these observed results.
Mate tea (Ilex paraguariensis) promotes satiety and body weight lowering in mice: involvement of glucagon-like peptide-1
We previously investigated the effects of an aqueous extract of maté (mate) tea, made from the leaves of Ilex paraguariensis, on the diabesity and metabolic syndrome features in a mouse model. Mate induced significant decreases in body weight (BW), body mass index, and food intake (FI). In this study, to verify the mode of action of mate on FI and consequently on BW, we examined the anorexic effects of mate on the appetite and satiety markers glucagon-like peptide 1 (GLP-1) and leptin in high-fat diet-fed ddY mice. GLP-1 is a peptide signal generated by the gastrointestinal tract, which regulates appetite and influences BW, whereas leptin is an afferent signal from the periphery to the brain in a homeostatic feedback loop that regulates adipose tissue mass, thus leading to decreased appetite and FI and increased energy expenditure.
Chronic administration of mate (50, 100 mg/kg) for 3 weeks significantly reduced FI, BW, and ameliorated blood fats, liver fats, and adipose tissue. Mate induced significant increases in GLP-1 levels and leptin levels compared with the control. Acute administration of major constituents of mate showed significant increases in GLP-1 levels by dicaffeoyl quinic acids and matesaponins, and significant induction of satiety by caffeoyl quinic acids and caffeine in ddY mice. These findings suggest that mate may induce anorexic effects by direct induction of satiety and by stimulation of GLP-1 secretion and modulation of serum leptin levels.

Mangifera indica
Reversal of Increase in Intestinal Permeability by Mangifera indica Seed Kernel Extract in High-Fat Diet-Induced Obese Mice
The present study demonstrated that MESK has the ability to reverse HFD-induced increases in intestinal permeability by upregulating the expression of key TJ proteins ZO-1 and Claudin-1 (Figure 15D and Figure 16D). We have also demonstrated that MESK and Orlistat increased the expression of Nesfatin-1 in the mouse jejunum (Figure 17C,D). Since Nesfatin-1 is a powerful satiety signal, a higher expression of this peptide hormone could be the reason behind the reduced weight gain (Figure 5A,B) observed in HFDO and HFDM groups, though the involvement of other satiety hormones cannot be ruled out. Orlistat acts by reducing the activity of intestinal lipase, and a recent study by Olszanecka-Glinianowicz has shown that long-term treatment (8 weeks) with Orlistat significantly increased the plasma levels of anorexigenic hormones glucagon-like peptide-1 (GLP-1) and Peptide YY (PYY) and the rise in the levels of these satiety hormone levels was twice those of the placebo group [42].

Momordica charantia
Role of GLP-1 in the Hypoglycemic Effects of Wild Bitter Gourd
This study aimed to examine the role of GLP-1 in the hypoglycemic activity of wild bitter gourd (Momordica charantia L., BG). In vitro, the GLP-1 secretion in STC-1, a murine enteroendocrine cell line, was dose dependently stimulated by water extract (WE), its fractions (WEL, >3 kD and WES, <3 kD), and a bitter compounds-rich fraction of BG. These stimulations were partially inhibited by probenecid, a bitter taste receptor inhibitor, and by U-73122, a phospholipase Cβ2 inhibitor.
These results suggested that the stimulation might involve, at least in part, certain bitter taste receptors and/or PLCβ2-signaling pathway. Two cucurbitane triterpenoids isolated from BG, 19-nor-cucurbita-5(10),6,8,22-(E),24-pentaen-3β-ol, and 5β,19-epoxycucurbita-6,24-diene-3β,23ξ-diol (karavilagenine E,) showed relative high efficacy in the stimulation. In vivo, mice fed BG diet showed higher insulinogenic index in an oral glucose tolerance test. A single oral dose of WE or WES pretreatment significantly improved intraperitoneal glucose tolerance. A single oral dose of WES significantly decreased glucose and increased insulin and GLP-1 in serum after 30 min. This acute hypoglycemic effect of WES was abolished by pretreatment with exendin-9, a GLP-1 receptor antagonist. Our data provide evidence that BG stimulates GLP-1 secretion which contributes, at least in part, to the antidiabetic activity of BG through an incretin effect.
Bitter gourd (Momordica Charantia L.) stimulates the secretion of the incretin Glucagon-like peptide-1 by enteroendocrine cells in vitro
The Glucagon-like peptide-1 hormone (GLP-1) is a key insulinotropic and glucagonostatic incretin produced by the L enteroendocrine cells (EECs) of the intestines. On the luminal side, EECs express a variety of taste receptors, among them, members of the bitter taste receptor family or taste receptors type 2 (TAS2Rs).
The fruit of the Momordica charantia is called bitter gourd (BG) and has a distinctive bitter taste. This edible vegetable, common in tropical regions, is also a folk medicine employed for the management of type-2 Diabetes.
Within the last decade, there has been increasing evidence on chemosensory activity of EECs, and how food tastants can affect the secretion of intestinal hormones, presumably, by interacting with sweet, umami, and/or bitter taste receptors. We hypothesized that the hypoglycaemic properties of BG (or a part of it) would be based on the stimulation of GLP-1 secretion by EECs, likely through interaction with TAS2Rs.
Here, we studied the GLP-1 secretion by in vitro EECs (mouse and human) when exposed to different BG specimens (genotypes and extracts). BG samples significantly induced GLP-1 secretion in mouse STC-1 and in human HuTu-80 EECs. Besides, TAS2R16 responded dose-dependently to BG extracts, suggesting its potential involvement in the interaction between BG and EECs. Based on the two EEC in vitro models, the GLP-1 secretion induced by different BG cultivars was correlated with antidiabetic effects as found in an in vivo piglet study. The role of GLP-1 secretion in BG’s antidiabetic effects and the difference between both cell-based models will be discussed.
GLP-I secretion in healthy and diabetic Wistar rats in response to aqueous extract of Momordica charantia
Background: Diabetes mellitus is one of the major global health disorders increasing at an alarming rate in both developed and developing countries. The objective of this study was to assess the effect of aqueous extract of Momordica charantia (AEMC) on fasting blood glucose (FBG), tissue glycogen, glycosylated haemoglobin, plasma concentrations of insulin and GLP-1 hormone (glucagon-like peptide 1) in healthy and diabetic wistar rats.
Methods: Male Wistar rats (both normal and diabetic) were treated with AEMC by gavaging (300 mg/kg body wt/day for 28 days).
Results: AEMC was found to increase tissue glycogen, serum insulin and GLP-1 non-significantly (P > 0.05) in normal, significantly (P < 0.01) in diabetic Wistar rats, whereas decrease in FBG and Glycosylated haemoglobin non-significantly (P > 0.05) in normal, significantly (P < 0.01) in diabetic Wistar rats. The elevation of GLP-1 level in normal and diabetic treated groups may be due to the L-cell regeneration and proliferation by binding with L-cell receptors and makes a conformational change, resulting in the activation of a series of signal transducers. The polar molecules of M. charantia also depolarize the L-cell through elevation of intracellular Ca2+concentration and which in turn releases GLP-1. GLP-1 in turn elevates beta-cell proliferation and insulin secretion.
Conclusion: The findings tend to provide a possible explanation for the hypoglycemic action of M. charantia fruit extracts as alternative nutritional therapy in the management and treatment of diabetes.

Myricetin
Myricetin: a potent approach for the treatment of type 2 diabetes as a natural class B GPCR agonist – PMC
The physiologic properties of glucagon-like peptide 1 (GLP-1) make it a potent candidate drug target in the treatment of type 2 diabetes mellitus (T2DM). GLP-1 is capable of regulating the blood glucose level by insulin secretion after administration of oral glucose. The advantages of GLP-1 for the avoidance of hypoglycemia and the control of body weight are attractive despite its poor stability. The clinical efficacies of long-acting GLP-1 derivatives strongly support discovery pursuits aimed at identifying and developing orally active, small-molecule GLP-1 receptor (GLP-1R) agonists. The purpose of this study was to identify and characterize a novel oral agonist of GLP-1R (i.e., myricetin). The insulinotropic characterization of myricetin was performed in isolated islets and in Wistar rats. Long-term oral administration of myricetin demonstrated glucoregulatory activity. The data in this study suggest that myricetin might be a potential drug candidate for the treatment of T2DM as a GLP-1R agonist.

Notoginsenoside Ft1
Increased glucagon-like peptide-1 secretion may be involved in antidiabetic effects of ginsenosides
Panax ginseng is one of the most popular herbal remedies. Ginsenosides, major bioactive constituents in P. ginseng, have shown good antidiabetic action, but the precise mechanism was not fully understood. Glucagon-like peptide-1 (GLP1) is considered to be an important incretin that can regulate glucose homeostasis in the gastrointestinal tract after meals. The aim of this study was to investigate whether ginseng total saponins (GTS) exerts its antidiabetic effects via modulating GLP1 release. Ginsenoside Rb1 (Rb1), the most abundant constituent in GTS, was selected to further explore the underlying mechanisms in cultured NCI-H716 cells. Diabetic rats were developed by a combination of high-fat diet and low-dose streptozotocin injection. The diabetic rats orally received GTS (150 or 300 mg/kg) daily for 4 weeks. It was found that GTS treatment significantly ameliorated hyperglycemia and dyslipidemia, accompanied by a significant increase in glucose-induced GLP1 secretion and upregulation of proglucagon gene expression. Data from NCI-H716 cells showed that both GTS and Rb1 promoted GLP1 secretion. It was observed that Rb1 increased the ratio of intracellular ATP to ADP concentration and intracellular Ca2+ concentration. The metabolic inhibitor azide (3 mM), the KATP channel opener diazoxide (340 μM), and the Ca2+ channel blocker nifedipine (20 μM) significantly reversed Rb1-mediated GLP1 secretion. All these results drew a conclusion that ginsenosides stimulated GLP1 secretion both in vivo and in vitro. The antidiabetic effects of ginsenosides may be a result of enhanced GLP1 secretion.
Notoginsenoside Ft1 acts as a TGR5 agonist but FXR antagonist to alleviate high fat diet-induced obesity and insulin resistance in mice
Obesity and its associated complications are highly related to a current public health crisis around the world. A growing body of evidence has indicated that G-protein coupled bile acid (BA) receptor TGR5 (also known as Gpbar-1) is a potential drug target to treat obesity and associated metabolic disorders. We have identified notoginsenoside Ft1 (Ft1) from Panax notoginseng as an agonist of TGR5 in vitro. However, the pharmacological effects of Ft1 on diet-induced obese (DIO) mice and the underlying mechanisms are still elusive. Here we show that Ft1 (100 mg/100 diet) increased adipose lipolysis, promoted fat browning in inguinal adipose tissue and induced glucagon-like peptide-1 (GLP-1) secretion in the ileum of wild type but not Tgr5−/− obese mice.
In addition, Ft1 elevated serum free and taurine-conjugated bile acids (BAs) by antagonizing Fxr transcriptional activities in the ileum to activate Tgr5 in the adipose tissues. The metabolic benefits of Ft1 were abolished in Cyp27a1−/− mice which have much lower BA levels. These results identify Ft1 as a single compound with opposite activities on two key BA receptors to alleviate high fat diet-induced obesity and insulin resistance in mice.

Peganum harmala
Peganum harmala enhanced GLP-1 and restored insulin signaling to alleviate AlCl3-induced Alzheimer-like pathology model
Peganum harmala (P. harmala) is a folk medicinal herb used in the Sinai Peninsula (Egypt) as a remedy for central disorders. The main constituents, harmine and harmaline, have displayed therapeutic efficacy against Alzheimer’s disease (AD); however, the P. harmalapotential on sensitizing central insulin to combat AD remains to be clarified. An AD-like rat model was induced by aluminum chloride (AlCl3; 50 mg/kg/day for six consecutive weeks; i.p), whereas a methanolic standardized P. harmala seed extract (187.5 mg/kg; p.o) was given to AD rats starting 2 weeks post AlCl3 exposure. Two additional groups of rats were administered either the vehicle to serve as the normal control or the vehicle + P. harmala seed extract to serve as the P. harmala control group. P. harmala enhanced cognition appraised by Y-maze and Morris water maze tests and improved histopathological structures altered by AlCl3.
Additionally, it heightened the hippocampal contents of glucagon-like peptide (GLP-1) and insulin, but abated insulin receptor substrate-1 phosphorylation at serine 307 (pS307-IRS-1). Besides, P. harmala increased phosphorylated Akt at serine 473 (pS473-Akt) and glucose transporter type (GLUT)4. The extract also curtailed the hippocampal content of beta amyloid (Aβ)42, glycogen synthase (GSK)-3β and phosphorylated tau. It also enhanced Nrf2, while reduced lipid peroxides and replenished glutathione. In conclusion, combating insulin resistance by P. harmala is a novel machinery in attenuating the insidious progression of AD by enhancing both insulin and GLP-1 trajectories in the hippocampus favoring GLUT4 production.

Pinus koraiensis
In Pasman’s study, Korean pine nut oil was administered in form of FFA (capsules with 3 g of FFA) or triglycerides (capsules with 3 g of TG). Olive oil was used as placebo (3 g). Subjects received capsules in combination with a light breakfast. 0, 30, 60, 90, 120, 180, and 240 minutes after administration the amount of released gut hormones CCK and GLP-1, peptide YY (PYY) and gastric hormone ghrelin was measured in the collected blood of the subjects. Immediately after each blood collection, the subjects completed 100 mm visual analogue scale (VAS), which is used for quantification of appetite sensations [7].
In comparison to placebo the release of CCK was significantly higher 30-180 minutes after Korean pine nuts FFA administration (by 60% higher than placebo 30 minutes after administration) and 60-120 minutes after TG administration (22%, 60 min.). In respect of GLP-1 release, the content of GLP-1 was by 25.1% higher 60 minutes after FFA administration compared to placebo. Levels of ghrelin and PYY did not differentiate between FFA, TG and placebo. No differences were also observed for hunger of desire to eat. 30 minutes after administration the feeling of fullness was lower for pine nut TG (not for FFA). Prospective food consumption (PFC), expressed by VAS, was by 36% lower 30 minutes after FFA administration (compared to placebo), administration of TG also seemed to lower PFC, but not so significantly [17].
Conclusion: PinnoThin™ shows satiating effects via CCK and GLP-1 release (acute effect on satiety during the food or immediately after the food). The induction of GLP-1 from the distal part of colon have higher importance for suppression of food intake after food due to the permanent feeling of satiety between the meals. Decrease of energy content of consumed food by 50 kcal is significant, because the average increase in body weight (in women population) corresponds to energy imbalance only 10 – 50 kcal per day (5).
The results show that 3 g of PinnoThin™ increase the release of CCK and GLP-1 for 3 hours. This may lead to a reduced prospective food intake suggesting that pine nut FFA and TG may act as an appetite suppressant in overweight women.

Prunus Africana
The aim of the study was to investigate the mechanism of the anti-diabetic properties of P africana extract. Increased insulin secretion was confirmed by the increased C- peptide concentration in plasma samples of rats treated with P. africana. In order to explain the high insulin levels, several hypothesis’ were investigated: (1) P. africana may increase insulin secretion in β cells, hence the effect of P. africana on insulin secretion by INS-1 cells was investigated; (2) P. africana may increase insulin secretion by prolonging the half-life of glucagon like peptide-1 (GLP-1) by decreasing dipeptidyl peptidase IV (DPP IV) activity.
In summary, this study indicates that P. africana is indirectly involved in inhibiting DDPIV. This in turn can increase the half life of GLP-1, which in turn can enhance the secretion of insulin. P. africana increases glucose utilization although there was no evidence that the GLUT 4 transporter has a higher expression in the plant treated rats. Further studies should be conducted to investigate the expression of GLUT1 under the same conditions.

Pueraria Montana Var. Lobata (Willdenow) Maesen
Puerarin ameliorates hyperglycemia in HFD diabetic mice by promoting β-cell neogenesis via GLP-1 R signaling activation
Background: Diabetes is characterized by β-cell loss and dysfunction. A strategy for diabetes treatment is to promote new β-cell formation. Puerarin is an isoflavone from the root of Pueraria lobata (Willd.) Ohwi. Our previous study demonstrated puerarin could ameliorate hyperglycemia in diabetic mice. However, related mechanisms and potential roles of puerarin in β-cell neogenesis have not been elucidated.
Purpose: The present study aims to investigate whether anti-diabetic effect of puerarin is dependent on promoting β-cell neogenesis via GLP-1 R signaling activation.
Methods: A high-fat diet (HFD) induced diabetic mouse model was applied to investigate effects of puerarin in vivo, exendin-4 (GLP-1 R agonist) and metformin were used as positive controls. Moreover, related mechanisms and GLP-1 R downstream signal transduction were explored in isolated cultured mouse pancreatic ductal cells.
Results: Puerarin improved glucose homeostasis in HFD diabetic mice significantly. Markers of new β-cell formation (insulin, PDX1 and Ngn3) were observed in pancreatic ducts of HFD mice treated by puerarin. Of note, efficacy of puerarin in vivo was suppressed by GLP-1 R antagonist exendin9-39, but enhanced by exendin-4 respectively. In cultured mouse pancreatic ductal cells, puerarin induced expressions of insulin and PDX1, upregulated GLP-1 R expression and activated β-catenin and STAT3 subsequently. Expressions of insulin and PDX1 in ductal cells could be blocked by exendin9-39, or β-catenin inhibitor ICG001, or JAK2 inhibitor AG490.
Conclusion: These data clarified puerarin ameliorated hyperglycemia of HFD mice via a novel mechanism involved promoting β-cell neogenesis. Our finding highlights the potential value of puerarin developing as an anti-diabetic agent.
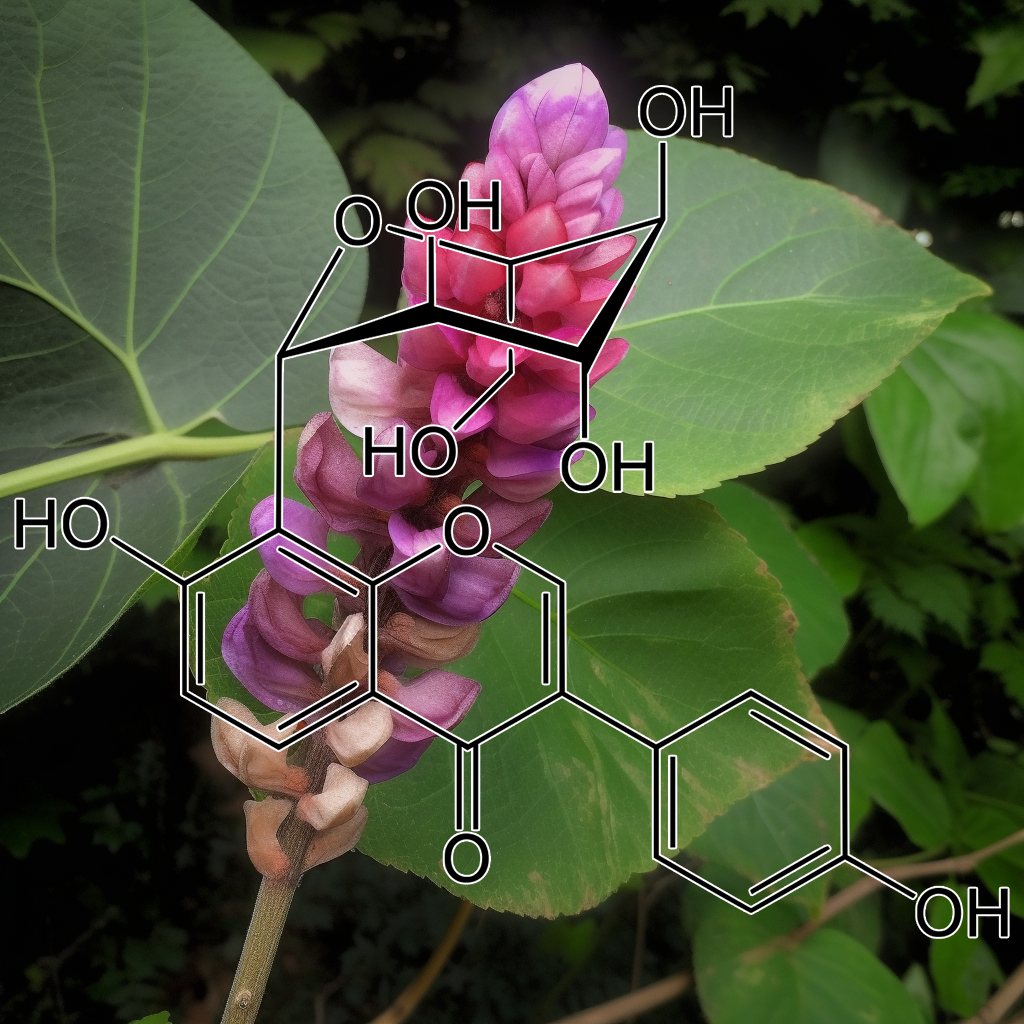
Puerarin
Molecular Mechanism of Puerarin Against Diabetes and its Complications
Enhance GLP-1R Signaling Pathway
A recent report showed that chronic hyperglycemia could lead to the loss of the glucagon-like peptide-1 receptor (GLP-1R) from the cell surface and impairment of GLP-1R signaling. Therefore, recovery of the GLP-1R expression itself and the GLP-1R signaling transduction might be a strategy for diabetic treatment.
On one side, it has been shown that Puerarin rescued the β-cell failure and promoted β-cell proliferation through up-regulating GLP-1R expression, which enhanced GLP-1R signaling and activated its downstream target protein kinase B (Akt), which led to the inactivation of forkhead box transcription factor O1 (Foxo1) and Caspase-3 subsequently. Foxo1 acts as a transcription factor to inhibit pancreatic duodenum homeobox-1 (PDX-1) activity and mediate-cell dysfunction and apoptosis. The caspase family of proteins is involved in inducing apoptosis.
On the other hand, Puerarin induced β-cell replication and neogenesis in pancreatic ductal cells of HFD mice depended on GLP-1R expression in ductal cells together with activating β-catenin and STAT3, subsequently activated Wnt/β-catenin and JAK2/STAT3, up-regulation of PDX-1 and Ngn3 expression, which up-regulation of TCFTL2 expression, that might be downstream effectors of the GLP-1R signaling cascade. Puerarin triggers the pancreatic ductal epithelial cell to β-cell conversion through activating GLP-1R/Wnt/STAT3 signaling cascade.
The pharmacological properties of Puerarin, a major active component of RP, have been recently uncovered. Puerarin has been shown to exert antidiabetic effects of reducing blood glucose and improving diabetes complications in patients. It has been proven to promote β-cell neogenesis and inhibit apoptosis, enhance the insulin receptor signaling, boost glucose transport and uptake, and suppress hepatic gluconeogenesis through multiple approaches, including activation of GLP-1R and PI3K/Akt signalings and inhibition of ROS production and Caspase/AIF apoptotic pathway in the pancreas, enhancement of GLUT4 delivery and PPAR receptor expression alongside increased fatty acid oxidation in skeletal muscle and adipose tissue, and PI3K/Akt activation in the liver.
Thus, insulin secretion is restored to improve IR to lower blood glucose. As for diabetic complications, Puerarin has been proven to significantly delay their occurrence and progression via eliminating excessive nonenzymatic glycosylation, oxidative stress, and inflammatory response and suppressing apoptosis caused by chronic hyperglycemia.
Puerarin Protects Pancreatic β-Cells in Obese Diabetic Mice via Activation of GLP-1 R Signaling
Diabetes is characterized by a loss and dysfunction of the β-cell. Glucagon-like peptide 1 receptor (GLP-1 R) signaling plays an important role in β-cell survival and function. It is meaningful to identify promising agents from natural products which might activate GLP-1 R signaling. In this study, puerarin, a diet isoflavone, was evaluated its beneficial effects on β-cell survival and GLP-1 R pathway. We showed that puerarin reduced the body weight gain, normalized blood glucose, and improved glucose tolerance in high-fat diet-induced and db/db diabetic mice. Most importantly, increased β-cell mass and β-cell proliferation but decreased β-cell apoptosis were observed in puerarin-treated diabetic mice as examined by immunostaining of mice pancreatic sections. The protective effect of puerarin on β-cell survival was confirmed in isolated mouse islets treated with high glucose.
Further mechanism studies showed that the circulating level of GLP-1 in mice was unaffected by puerarin. However, puerarin enhanced GLP-1 R signaling by up-regulating expressions of GLP-1 R and pancreatic and duodenal homeobox 1, which subsequently led to protein kinase B (Akt) activation but forkhead box O1 inactivation, and promoted β-cell survival. The protective effect of puerarin was remarkably suppressed by Exendin(9–39), an antagonist of GLP-1 R. Our study demonstrated puerarin improved glucose homeostasis in obese diabetic mice and identified a novel role of puerarin in protecting β-cell survival by mechanisms involving activation of GLP-1 R signaling and downstream targets.
Quercetin
Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus
Diabetes Mellitus (DM) is a metabolic disorder that is spreading alarmingly around the globe. Type-2 DM (T2DM) is characterized by low-grade inflammation and insulin resistance and is closely linked to obesity. T2DM is mainly controlled by lifestyle/dietary changes and oral antidiabetic drugs but requires insulin in severe cases. Many of the drugs that are currently used to treat DM are costly and present adverse side effects. Several cellular, animal, and clinical studies have provided compelling evidence that flavonoids have therapeutic potential in the management of diabetes and its complications.
Quercetin is a flavonoid, present in various natural sources, which has demonstrated in vitro and in vivo antidiabetic properties. It improves oral glucose tolerance, as well as pancreatic β-cell function to secrete insulin. It inhibits the α-glucosidase and DPP-IV enzymes, which prolong the half-life of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Quercetin also suppresses the release of pro-inflammatory markers such as IL-1β, IL-4, IL-6, and TNF-α. Further studies are warranted to elucidate the mode(s) of action of quercetin at the molecular level. This review demonstrates the therapeutic potential of quercetin in the management of T2DM.
Quercetin is a flavonoid, present in various natural sources, which has demonstrated in vitro and in vivo antidiabetic properties. It improves oral glucose tolerance, as well as pancreatic β-cell function to secrete insulin. It inhibits the α-glucosidase and DPP-IV enzymes, which prolong the half-life of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Quercetin also suppresses the release of pro-inflammatory markers such as IL-1β, IL-4, IL-6, and TNF-α.

Radix Ophiopogonis
Antidiabetic activities of oligosaccharides of Ophiopogonis japonicus in experimental type 2 diabetic rats
The aim of the present study is to investigate the antidiabetic properties of oligosaccharides of Ophiopogonis japonicus (OOJ) in experimental type 2 diabetic rats. OOJ was administered orally in doses of 225 and 450 mg/kg body weight to high-fat diet and low-dose streptozotocin (STZ)-induced type 2 diabetic rats for 3 weeks. The results showed that OOJ treatment could increase body weight, decrease organ related weights of liver and kidney, reduce fasting blood glucose level, and improve oral glucose tolerance in diabetic rats.
Moreover, increased glycogen content in liver and skeletal muscle, reduced urinary protein excretion, higher hepatic GCK enzyme activity, lower hepatic PEPCK enzyme activity, enhanced GLP-1 level, decreased glucagon level and alleviated histopathological changes of pancreas occurred in OOJ-treated diabetic rats by comparison with untreated diabetic rats. This study demonstrates, for the first time to our knowledge, that OOJ exerts remarkable antidiabetic effect in experimental type 2 diabetes mellitus, thus justifying its traditional usage.

Rehmanniae Radix
Extracts of Rehmanniae radix, Ginseng radix and Scutellariae radix improve glucose-stimulated insulin secretion and β-cell proliferation through IRS2 induction
Treatment with exendin-4, a GLP-1 agonist, enhances glucose-stimulated insulin secretion in rodents and humans. Human studies have shown that extended administration of GLP-1 not only augments glucose-stimulated insulin secretion, but also shifts the dynamics of the insulin response to earlier release in both diabetic and non-diabetic humans [2,9]. The mechanism was revealed as acting directly through the cAMP/protein kinase A (PKA) pathway to enhance and sensitize β-cells to glucose-stimulated insulin secretion. Activated PKA may close KATPchannels, and activate Ca2+ channels and exocytosis of insulin containing granules [3]. GLP-1 also increases insulin secretion in a calcium- and PKA-independent manner by mobilizing secretory vesicles to enter the readily releasable pool [6]. R. radix, Ginseng radix and S. radixmay enhance glucose-stimulated insulin secretion through cAMP/PKA-dependent and/or -independent pathways.

Resveratrol
Probiotic and resveratrol normalize GLP-1 levels and oxidative stress in the intestine of diabetic rats
Background: Recently, the use of incretins has been considered as a therapeutic target for diabetes. One of the important incretins in the improvement of diabetes is glucagon-like peptide (GLP-1), which is secreted by the gut and reduces the apoptosis of pancreatic β-cells and improves insulin sensitivity. In this experiment we determined the effects of resveratrol and probiotics on insulin resistance, oxidative stress, and GLP-1 in type 2 diabetes (T2D) rats.
Methods: In this study, 40 male Wistar male rats were divided into 5 groups: 1. Control group, 2. T2D, 3. T2D treated with probiotics, 4. T2D treated with resveratrol, 5. T2D group treated with probiotics and resveratrol. After four weeks, the intestine were removed for histopathological analysis, biochemical tests, and oxidative stress markers.
Results: Probiotics and resveratrol significantly decreased (p < 0.001) glucose and insulin resistance, and increased (p < 0.001) GLP-1 and total antioxidant capacity compared to the diabetic group. Treatment with probiotics and resveratrol also returned intestinal histological changes in diabetic rats to normal.
Conclusion: Resveratrol and probiotics appear to be effective in controlling T2D by increasing GLP-1 levels and reducing oxidative stress.

Rhizoma Coptidis
Effect of Rhizoma coptidis (Huang Lian) on Treating Diabetes Mellitus
RC was mainly composed of a diversity of alkaloids, including berberine (6.88% to 13.64%), palmatine (1.28% to 2.12%), jateorrhizine (0.77% to 1.32%), coptisine (0.42% to 0.85%), epiberberine (0.42% to 0.92%), worenine, and magnoflorine, all of which are considered to be its active components [22]. Berberine (BBR), an isoquinoline alkaloid, is the major active component of RC. Modern pharmacological researches have showed multiple mechanisms of BBR to lower blood glucose, such as improvement of insulin sensitivity, increase of insulin secretion [23], promotion of intestinal glucagon-like protein-1 (GLP-1) secretion [24], inhibition of hepatic gluconeogenesis [25], induction of glycolysis in peripheral tissues [26], promotion of antioxidant activities [16, 17], regulation of lipid disorders [27], and modulation of the gut microbiota [28, 29]. Lee et al. [30] showed that BBR improved insulin sensitivity and increased insulin secretion possibly through activating adenosine monophosphate-activated protein kinase pathway (AMPK) activity.

Silymarin
Silymarin protects against high fat diet-evoked metabolic injury by induction of glucagon-like peptide 1 and sirtuin 1
Results showed that silymarin intervention from 0 week or 8th week of the time course in the experiment significantly improved metabolic syndromes, including insulin resistance and dyslipidemia in high fat diet-treated rats. Silymarin also can ameliorate the HFD-impaired glucose tolerance and recover, further enhance HFD-impaired glucagon-like peptide 1 (GLP-1) secretion in these rats.
Intervention of silymarin from 0 week can prevent HFD-induced hepatic steatosis, pancreas fibrosis, and decreased sirtuin 1 protein levels of pancreas and liver in rats. In conclusion, silymarin can prevent and ameliorate HFD-induced metabolic syndrome, as well as improvement of GLP-1 secretion and Sirt1 protein expression, suggesting that silymarin may be a potential food factor against metabolic injury.

Smallanthus Sonchifolius
Yacon (Smallanthus sonchifolius) as a Food Supplement: Health-Promoting Benefits of Fructooligosaccharides
Several preclinical and clinical trials have shown that yacon root FOS have a notable hypoglycemic effect. In an experiment using streptozotocin-induced diabetic rats, the number of insulin-positive pancreatic cells and glucagon-like peptide-1 (GLP-1) significantly increased, while visceral abdominal fat was reduced and fasting insulin serum levels were slightly increased in diabetic rats supplemented with yacon flour (340 or 6800 mg FOS/kg body weight (bw).) for 90 days [54]. In another study using Zucker fa/fa male rats, yacon at 6.5% in chow reduced blood glucose levels and improved hepatic insulin sensitivity. In this case, dietary yacon significantly reduced Trb3 hepatic expression and increased Akt expression, improving insulin sensitivity in the liver [55].
It has also been shown that dietary FOS are able to increase the secretion of peptides by the gastrointestinal diffuse neuroendocrine system via SCFA production, acting as modulators of appetite and increasing satiety [78]. The physiological control of satiety is partly regulated by intestinal peptide secretion including cholecystokinin (CCK), PYY and GLP-1. It is noteworthy that this regulation is complex and involves a range of mechanisms and multiple control systems [79]. Nevertheless, SCFA can directly increase PYY and GLP-1 secretion by Ffar1 and Ffar2 activation in the colon [80]. Conversely, long-term studies have suggested that a long exposure time is needed for the intestinal microbiota to adapt and produce the amounts of SCFA to elicit the physiological effect of satiety. Increased gut motility may also be affected by intestinal peptide secretion [81]. However, SCFA such as butyrate are able to exert direct effects on myenteric neurons and increase the intestinal motility, supporting the hypothesis by which a high fiber intake accelerates the colonic transit [82].

Tithonia Diversifolia
TGR5 potentiates GLP-1 secretion and cause pancreatic islet regeneration in response to Tithonia diversifolia saponin-rich extract in diabetic model mice
Background: Activation of Takeda G-protein bile acid receptor 5 (TGR5) secretes glucagon-like peptide-1 (GLP-1) downstream insulin release. Saponin which has been indicated as the antidiabetic principle in plant shared similar steroidal scaffold with cholic acid (primary agonist of TGR5), hence antidiabetic mechanism of Tithonia diversifolia leaves saponin-rich extract (TDS) was investigated via TGR5/GLP-1 pathway.
Methods: Insulin secretion and expression of GLP-1 were measured in MIN6 β-cells incubated with TDS and cholic acid a known agonist of TGR5 at (25, 75, 125, 325) µg/ml and treatment were compared to incubation with insulin secretagogue metformin (50 mM). The action of TDS on TGR5 was studied in vivo using swiss albino mice.
Results: β-cell assay showed dose dependent increase in insulin secretion/1 × 106cells incubated with TDS (25, 75, 125, 325 µg/ml) when compared with positive control (50 mM metformin) and vehicle control (Cholic acid). TDS increased GLP-1 and insulin expression and decrease the percentage of%HbA1c in treated mice when compared with control (p < 0.05). TGR5 gene were highly expressed in cell incubated with 20–60 mg/kg body weight of TDS compared to STZ-induced diabetic mice and immunoblotting of TGR5 protein showed higher expression in all groups treated with TDS compared to STZ-induced diabetic mice.
Conclusion: TDS exhibit its antidiabetic mechanism via TGR5 agonism leading to increase GLP-1 expression and subsequently insulin release. Hence TDS may represent a new therapeutic strategy for type-2 diabetes, a finding which could be further investigated in clinical trials.

Zein Hydrolysate
GLP-1 secretion is enhanced directly in the ileum but indirectly in the duodenum by a newly identified potent stimulator, zein hydrolysate, in rats
Glucagon-like peptide-1 (GLP-1) is released from enteroendocrine cells (L cells) in response to food ingestion. The mechanism by which dietary peptides stimulate GLP-1 secretion in the gut is unknown. In the present study, we found that a hydrolysate prepared from zein, a major corn protein [zein hydrolysate (ZeinH)], strongly stimulates GLP-1 secretion in enteroendocrine GLUTag cells. Stimulatory mechanisms of GLP-1 secretion induced by ZeinH were investigated in the rat small intestine under anesthesia. Blood was collected through a portal catheter before and after ZeinH administration into different sites of the small intestine. The duodenal, jejunal, and ileal administration of ZeinH induced dose-dependent increases in portal GLP-1 concentration. GLP-1 secretion in response to the ileal administration of ZeinH was higher than that in the duodenal or jejunal administration. Capsaicin treatment on esophageal vagal trunks abolished the GLP-1 secretion induced by duodenal ZeinH but did not affect the secretion induced by jejunal or ileal ZeinH.
These results suggest that ZeinH in the jejunum or ileum directly stimulates GLP-1 secretion but duodenal ZeinH indirectly stimulates GLP-1 secretion via the vagal afferent nerve. A direct blood sampling method from the duodenal vein and ileal mesenteric vein revealed that ZeinH administered into the ligated duodenal loop enhanced GLP-1 concentration in the ileal mesenteric vein but not in the duodenal vein. This confirmed that ZeinH in the duodenum induces GLP-1 secretion from L cells located in the ileum by an indirect mechanism. These results indicate that a potent GLP-1-releasing peptide, ZeinH, induces GLP-1 secretion by direct and indirect mechanisms in the rat intestine.
function force_product_description_display() {
global $product;
if ( $product ) {
echo ‘
‘;
}
}
add_action(‘woocommerce_after_single_product_summary’, ‘force_product_description_display’, 5);
| Size | 25g, 100g |
|---|
12 reviews for GLP-1 ACTIVATOR
Related products
-
INTERSTELLAR SPICE (Polyphenol Powerhouse)
Rated 5.00 out of 55.0 $75.00 – $275.00Price range: $75.00 through $275.00








Melissa Volz –
GLP-1 Activator
I ordered this because I have a hard time snacking late in the day. I am an all or nothing kind of person. I feel like if I’m going to fast I’m going to fast for several days which I think may have caused me some trouble in the past because I would fast and lose weight and then overeat after couple of months and the weight would come back. The same 30 pounds. A problem I feel many women have. So going to try shorter fasts on daily basis so 22/2 here I am. I have been taking this for 4 days now as instructed. 1/2 tsp in the morning and then 1/8tsp every 3-4 hours. I have maintained working out and eating whatever my family is eating in a two hour window. Which has been home cooked meals mostly carnivore with some fruit and nuts mixed in a couple of days. Today my went to my parents and they made my favorite meal (homemade mashed potatoes BBQ ribs chicken and green beans) I thought uh oh I’m really going to have to focus and stay strong and I did not have the slightest craving to over indulge in my food. I stuck with the meats. I couldn’t even finish all my food knowing I needed to probably eat more. But probably ended up eating half of what I normally would. I really expected to feel stuffed or uncomfortable but I felt nothing like that. It was like I just stopped eating I assume because I was actually full without having that feeling that I need to clean my plate. I was not even tempted by my mom’s delicious Texas Sheet cake and ice cream. So far in 4 days I am down 7 pounds. Amazing to see!!! I can see changes already and I’m loving it. I will definitely be ordering more when I get low. I have also been able to avoid snacking on goodies that have been brought to work. 12 hour shifts with birthday cakes and crumble cookie and I was not tempted at all. Didn’t even give it a second thought. I also feel as though I have a drive to be productive throughout the day. I’m on a mission. Gavin was also so kind as to include a sample of Obesogen which I will be starting this coming week to see how I feel with it. What a kind a generous man. Thanks Gavin for all of your dedication to helping people be the best self of them they can be.❤️😊
Melissa V14
DL –
I was trying to hold off leaving a review for a couple of days, or at least a week, but today was my first day trying the GLP activator & I am just blown away how I’ve felt all day. Usually I can make it to 15-16 hours fasted (not easily) and that’s it. Today I am going on 20 hours and I don’t even have the slightest urge to eat anything!!! Another test will be going to a big party this evening – normally I am a glutton for wine (and I know it will be flowing there) so I am curious to see how I react.
So excited about this!!
Jackie Rae –
The GLP-1 activator !!!!
The FIRST day I tried this, I fasted 22/2 and in my feeding window I tried to eat a small very low carb meal, and I could only eat half of it !!!! It truly DID keep the hunger waves at bay during fasting, I am losing body fat steadily and so excited to receive my GAINZ blend this week and get started adding some lean mass as well. This blend is a must have if you struggle with hunger/nausea and lethargy during fasting and atop the other blends I have purchased it’s like icing on CAKE !!!! Gavin has yet another masterpiece in this GLP blend, I’m a week in, and stoked about the results I’ve seen so far and looking forward to hitting the goal I’ve set for myself and then using the blends for support in maintenance !!! Thanks again, Gavin <3
Jackie Rae
Kali Dass –
So I need to share my experience using the new GLP 1 product because it absolutely blows my mind that this product works so quickly and so well and in my personal experience, without any negative side effects.
I got my samples of the product just last week and used only one dose the following two days, then because my next couple of days got a little crazy, I forgot to take it over the weekend.
Now, here I am two days later. Today my daughter walked into my house and said to me, “Wow! Look at you skinny mini. You look like you lost weight.” I honestly hadn’t realized it. The kicker here is that I haven’t used the product again (yet) and my appetite is still suppressed. Remember, this is only a single dose for two days in a row! Like what!?!
The thing is, I’m an afternoon and evening eater. I function better when I don’t eat until about 1pm. Even doing my best to follow the 22/2 intermittent fasting protocol, I really struggle in the evening around 5pm-6pm even if I’ve eaten at 1pm or so and especially since I’ve also been working on parasite cleansing over the last couple months using my own personal kitchen sink method. FYI Those little buggers are tough to kill and really make you crave the things that aren’t good for us.
I just want others to know about my experience bcz it might inspire you to give it a try, too. Using less product is a bonus, of course, but even with not eating much of anything all day long, I don’t have low blood sugar crashes leaving me feeling tired, cranky, run down or spaced out.
Mehak Waraich –
Gavin you killed it with this GLP, it has been a game changer for me – fasting is easier, appetite and cravings gone. I take autophagy so my appetite was under control but I still got these “cravings” that were more like obsessive thoughts about crap foods I loved. They call it “food noise” and let me tell you, I still got these noises in my head and had to fight myself against my own thoughts. I had less of these thoughts within days of starting GLP and now 3 weeks in, they are gone and my mind feels so free. Hardest part for me has always been fasting and feeding my little kids – now I barely notice that I’m feeding them food and have no desire to finish off leftovers.
I get full faster, stay full longer, have no cravings, and eating in general just feels more natural and intuitive. As someone who binge ate since her teen years, I never thought I could have a healthy relationship with food no matter what. I’m so thankful for these blends!
Bernadette Andersen –
GLP is down right mind blowing – I am a 62 year old health and wellness coach. Why do we become coaches: Because we have a hell of a health journey.
Most people suffer from constipation. I’m the opposite. I had surgery 7 years ago on my pancreas and it screwed up my digestion . I can have a bowel movement during my fasting up to 6 times a day. Food goes right through me. I know a little TMI. I would then get into to my eating window feeling completely ravished. Once I started eating I would have a terrible time stopping. It felt I would just stuff myself. I knew all that behavior wasn’t healthy at all. Not good for my digestion to eat to much in such a short window.
I also would do great fasting except when I watched my grandkids, I would get in there house at 7 am and was instantly hunger from all the kid energy.
I took GLP first day 1/4 tsp in am and afternoon. OMG!!!! no hunger at all. I would break my faster with my keifer as usual and couldn’t finish it. The day with grandkids, no problem, I did great
Once I did eat. I could eat slow, eat just enough and not over eat at all
It wasn’t about losing weight for me, it was about mindset, portion control, and getting out of an addictive behavior and balance in my life and not feeling bad about myself
Thank you again!!!!! Gavin and the interstellar team
Tamala Bell –
Here’s my take on Gavin’s GLP-1 blend💥💥💥 !
Here’s some backstory. I began taking Semaglutide on 11/2022. It was all the rage; people were losing weight super fast (or so I believed). So I went to an esthetician to begin the process of taking it. The initial dose was $150 (including consultation and lab work). It worked extremely well for one month, but the price was outrageous. So I had to go to my doctor to get new prescriptions because it was costing $75 per shot after a month of being on it through the esthetician (you took the shots weekly, $300 for the month)! So I went to see my doctor; the cost was $125 for eight weeks. I did that for 4 months until my body became accustomed to it, and I had to increase the dose for it to continue to work. For 8 weeks, the new medication cost $253. I was still losing weight, but extremely slowly because I was insulin resistant.
Here are all of the negative effects of that medicine that I experienced: extremely fatigued all day, constipation, headaches, unable to sleep, when taking the shots I would have itching or rash at the injection site, bloating, rash, constant acid burps,mood swings, irritability, wet fart when you thought they were regular ones, extreme dry mouth, I could NEVER quench my thirst, dry eyes, major muscle loss, which was my fault because I wasn’t working out because I didn’t have the energy to do so, and the kicker FOR ME was hair loss (because I dealt with all of the other issues for 10 months)! The hair loss scared me so much that I had to stop taking it, but I had a full vial (still in my refrigerator expired…$253 down the drain, but my health is far more important; it took me 10 months to figure that out.) that I had just paid for when Gavin came out with GLP-1, but I couldn’t subject myself to this medication any longer. So I bought one of his samples of the GPL-1, and it worked like day two, but I still had the Semaglutide in my system (I have read that it takes a month or so to leave your system). I’ve heard terrible stories about people stopping Semaglutide and their appetite returning 10 times stronger than it was before, and they reported they gained their weight back plus more! Now it’s been well over a month since I’ve been on GLP-1, and my appetite has not returned. I still felt the same when I took the Semaglutide, but with NO SIDE EFFECTS…NONE 🚫!
Gavin’s GLP-1 is far SUPERIOR to Semaglutide in every possible way, and it is far more affordable. Gavin’s is all natural, and it does not have crap in it like the Semaglutide that’s on the market! I have no appetite, feel fuller longer, I’m no longer bloated, I can fast longer, I am not tired, and when I eat, it’s impossible to finish all of my food (no longer part of the “clean your plate club”) there are no acid burps or wet farts, I am sleeping a little longer (6 hours), and I have energy. I have actually started working out. I actually loaded half a teaspoon for 5 days to get it into my system, and then I started breaking it down to 1/4 and now to 1/8 of a teaspoon. I take it once or twice a day and am perfectly fine. I am able to stick to my 22/2 intermittent fasting, and I feel fantastic! Also, when I’m on the go, I just take 1/8 tsp under my tongue, and I’m out the door!
I am so glad Gavin created this blend because I am in several Semaglutide Facebook groups where people are having terrible side effects from Semaglutide! People are vomiting uncontrollably, experiencing diarrhea, hair loss, severe rash, pancreatitis, and some serious stomach conditions, and some folks have filed a couple of class action lawsuits against the major Semaglutide manufacturers.
For the first time in fifteen years, I am three pounds short of achieving my goal. This is another high-quality blend (as are all of his blends) that I will keep in my cabinet. I can’t wait until he releases larger bags!
Keep doing what you’re doing, my brother from another mother❤, when it comes to developing these off-the-chain, high-quality🔥💥 blends that help your clients and family with their illnesses and diseases so that we can continue to heal ourselves.
This blend is PHENOMENAL🔥🔥🔥 !!!!
Rich Ryan –
I have trouble with late-night munchies. I can fast all day no problem, but then I eat for 2 hours on my 22/2, and have trouble stopping. On bad nights, I’ll sometimes stay up ’til dawn, munching on something the whole time. Bad Habit!
Since this is the case, I decided to focus my big dose of GLP-1 in the evenings. The first day, I didn’t notice much and ate the regular amount. The 2nd day, I didn’t really feel any different, but then I noticed I was full after finishing only half my dinner. I forced myself to eat the rest as I didn’t want to save it ’til the next day, but afterwards I felt like a waddling penguin trying to find the ice box. The next evening was the same. I was SO full, SO much quicker, that I had to struggle to finish my dinner.
Now, I’m having to cook smaller dinners so I don’t feel like an enormous waddling bag of fat that can’t move after I eat a moderate dinner. This stuff REALLY cuts the appetite. My dinner size has been cut in half.
I’ve also had no problems with late-night munchies since starting GLP-1 (it’s been a week). And actually I’ve been going to bed a couple of hours earlier than usual, as I’m not wasting time struggling with munchies and eating raw almonds, carrots and celery trying to cope.
If you’re trying to diet and lose weight, or just maintain the weight that you’ve already lost, then I have to say, GLP-1 is a MUST HAVE. It will make losing and maintaining body weight MUCH easier.
Another stellar blend by Interstellar!
Great Job!
Chris –
THIS BLEND WILL DESTROY THE BIG PHARMA DIET INDUSTRY! I have been water fasting and semi dry fasting very consistently for the past 2 years. I have always done some kind of fasting over the last 7 years thanks to GAVIN and his advice. Usually I am forced to deal with the hunger, the cravings, the stomach pangs, and all the ingestion and negative effects that occur when my body is detoxing from added chemicals and artificial highly processed forms of sugar NOT THIS TIME AND NEVER AGAIN.
I have been taking this blend for a few days now. The first dose I took about an hour later I went to the grocery store to get some steps in. HUNGER NEVER CROSSED MY MIND ONCE. I was also already 4 DAYS DEEP into water fasting at this point.
Later in the day I was supposed to meet my family for dinner and I thought to myself after I took my 3rd dose of GLP-1, what a great way to CHALLENGE myself and exercise EXTREME amounts of WILLPOWER, SELF CONTROL AND DISCIPLINE. I watched everyone eat and had ABSOLUTELY NO DESIRE TO EAT ANYTHING. I am currently on Day 6 of 7 of my water fast, who knows I might decide to do 10. 😌
GLP-1 is a gift from the UNIVERSE and THE INTERSTELLAR PLAN has delivered.
👑🐐🔥🔥🔥🔥🔥🔥
Jeremiah Burns –
I’ve used interstellar blends for over 5 years now and get ecstatic when Gavin creates a new masterpiece and GLP-1 is exactly that! I’ve tried every blend he’s created and usually add it to my regimen when he does, and GLP is on my list!
As someone in holistic care as a massage therapist I’ve heard the horror stories of the synthetic drug Ozempic, from stomach paralysis to complete kidney failure! The market was in need of a holistic fix and Gavin has met the challenge!
I tend to stack my blends to maximize the results and adding GLP to glucose blocker, hunger decimator, and anti-adipose has created the ultimate weight loss fasting combo.
If your looking to drop some pounds get GLP, if your looking for incredible fasting capabilities get the combo I recommended above and fast with ease!
Molly Danenhower (verified owner) –
I have been an Interstellar customer for several years and have experienced the improvements in health and vitality. After reading about the success of GLP-1 as an anti-diabetic, weight loss agent, I was very eager to try. I hoped it would help me manage night time cravings. When it arrived I asked my elderly mom to follow the regime with me. She had put on a substantial amount of weight and any attempt to lose it was unsuccessful. I was literally watching her health spiral downward.
We have now been taking GLP-1 for over a week and I am mind-blown by the results. Within 3 days my compulsion to snack GONE. But what is simply amazing is what this has done for my mother. By adding GLP-1 to her coffee, 3-4 times a day her eating habits have drastically changed. Her snack drawer ( full of sugary garbage) has been untouched …her uncontrollable cravings gone! The junk food has lost its appeal. She stopped taking her mid morning nap. I’m seeing a return of energy and the weight falling off. She asked if I would join her to restart a walking program that she hasn’t done in almost 3 years. I’m overwhelmed with gratitude.
I encourage anyone considering GLP-1 to just try it. It’s not an exaggeration to describe this as life changing.
Thank you Gavin for putting your energy and wisdom into healing and teaching people that good health is possible without Big Pharma. God bless you.
Shiva B –
I’ve shed 5lbs in my first two weeks using this blend exclusively WHILE continuing to build muscle… I am BLOWN AWAY by how well this is working and I confess I’ve only been consistent with a half daily recommended dose after the loading phase. In other words, this blend REALLY works !! For those of us who need rid ourselves of excess body fat and get control of bad habits in daily indulgences of excess eating, GO GO GO begin utilizing this blessing of a blend ASAP!!! Thank you Gavin for making GLP-1 Activator available for us who a ready for a REAL change!